Abstract
In most inherited red blood cell (RBC) disorders with high gene frequencies in malaria-endemic regions, the distribution of RBC hydration states is much wider than normal. The relationship between the hydration state of circulating RBCs and protection against severe falciparum malaria remains unexplored. The present investigation was prompted by a casual observation suggesting that falciparum merozoites were unable to invade isotonically dehydrated normal RBCs. We designed an experimental model to induce uniform and stable isotonic volume changes in RBC populations from healthy donors by increasing or decreasing their KCl contents through a reversible K+ permeabilization pulse. Swollen and mildly dehydrated RBCs were able to sustain Plasmodium falciparum cultures with similar efficiency to untreated RBCs. However, parasite invasion and growth were progressively reduced in dehydrated RBCs. In a parallel study, P falciparum invasion was investigated in density-fractionated RBCs from healthy subjects and from individuals with inherited RBC abnormalities affecting primarily hemoglobin (Hb) or the RBC membrane (thalassemias, hereditary ovalocytosis, xerocytosis, Hb CC, and Hb CS). Invasion was invariably reduced in the dense cell fractions in all conditions. These results suggest that the presence of dense RBCs is a protective factor, additional to any other protection mechanism prevailing in each of the different pathologies. (Blood. 2005; 105:4853-4860)
Introduction
Invasion of human red blood cells (RBCs) by Plasmodium falciparum merozoites is a complex, multistage process involving proximity reactions,1 contact, reorientation, secretion, and internalization events,2,3 the molecular nature of which is currently the subject of intense research.4-6 An important strategy for protection against falciparum malaria is based on reducing the fraction of RBCs vulnerable to parasite invasion, with the consequent decrease in the incidence of high parasitemia and severe malaria.2,3,7,8 This strategy is apparent in the most severe hemoglobinopathies (hemoglobin [Hb] EE, Hb CC, Hb H disease), in the homozygous forms of α- or β-thalassemia, and in certain inherited RBC membranopathies. The work presented here shows that decreased RBC volume, or increased density, reduces the infectivity of normal and abnormal RBCs by P falciparum, regardless of cell age, and suggests that this hydration effect may provide an additional level of protection in a variety of inherited RBC abnormalities predominant in malaria endemic regions.
The investigation arose from an unexpected observation. Heparinized blood from a healthy volunteer was kept at 4°C. Fresh RBCs from this reserve were used to initiate, and subsequently sustain, a P falciparum culture. Daily parasite counts remained satisfactory for the first 6 days but decreased sharply afterward, in contrast with the minor variations observed with RBCs from blood stored in citrate-dextrose at 4°C, in which extracellular [Ca2+] is largely reduced by chelation. Microscopic observation of the culture at the time the parasite count declined sharply revealed the appearance of a new subpopulation of dehydrated and crenated RBCs, which could be traced to recently added RBCs from the heparinized blood reserve because they shared the exact same appearance. The crucial observation was that merozoites could be seen attached to the dehydrated cells, but never internalized. The parasites had invaded only the few morphologically normal, discoid RBCs remaining from earlier RBC additions from the same reserve. This indicated that the sharp fall in parasitemia resulted from the failure of merozoites to invade the recently added, dehydrated cells. Further investigation of this process indicated that dehydration of the cold-stored cells resulted from inhibition of the plasma membrane Ca2+ pump (PMCA) by the low temperature.9 The consequent elevation of the intracellular free Ca2+ concentration activated Ca2+-sensitive K+ channels (Gardos channels,10,11 IK1,12 hSK413 ) triggering dehydration by net loss of KCl and water. Although the elevated Ca2+ would be immediately extruded from the cells as soon as they were suspended in the culture medium at 37°C, the dehydrated condition of human RBCs reverses very slowly.14 Besides the cautionary message not to cold-store RBCs intended for use in malaria cultures without reducing the calcium ion concentration in the medium, the unexpected and important observation here was that merozoites appeared unable to invade dehydrated RBCs.
In this paper we report the results of 2 studies aimed at characterizing the effects of RBC hydration state on P falciparum invasion and growth. These parameters were assessed in cultures sustained with RBCs of different volumes and densities. In the first study, the volume of normal RBCs was altered experimentally in isotonic conditions. In the second study, cultures were sustained with RBCs of different densities obtained by density separation of blood samples from healthy subjects and from patients with different inherited RBC abnormalities. The results showed that invasion efficiency is reduced in dehydrated RBCs and completely inhibited in profoundly dehydrated RBCs, whether the dehydrated state is experimentally induced or inherent in the particular density distribution of the normal or abnormal RBCs. The relevance of these findings to the assessment of the factors that determine the clinical severity of P falciparum malaria and to the selective pressures on individuals with RBC mutations in malaria endemic regions is discussed.
Materials and methods
Chemicals and solutions
The name and composition of the solutions was as follows (in mM): Wash solution, “W”: NaCl, 145; KCl, 2; HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-Na, pH 7.5 at 37°C, 10; and MgCl2, 0.15. Low-K+ solution, “LK”: NaCl, 135; KCl, 2; NaSCN, 10; HEPES-Na, pH 7.5 at 37°C, 10; and MgCl2, 0.15. High-K+ solution, “HK”: KCl, 137; NaSCN, 10; HEPES-Na, pH 7.5 at 37°C, 10; and MgCl2, 0.15. Solutions LK and HK were used alone or in combination to generate K+ concentrations from 2 to 137 mM, as indicated in Table 2 and the figures. Unless indicated otherwise, the final concentrations of added solutes were (in mM): EGTA (ethylen glycol-bis(aminoethylether)-tetraacetic acid), 1; CaCl2, 0.03; inosine, 5; ionophore A23187 (from 2 mM stock in ethanol), 0.01 (in the cell suspension). All additions were done from stock solutions at least 100-fold more concentrated than in the final solutions or cell suspensions. All chemicals were analytical reagent quality. EGTA, HEPES, glucose, inosine, dimethyl sulfoxide, RPMI 1640 medium (R0883), gentamicin sulfate, glutamine, sorbitol, and valinomycin were from Sigma (Poole, United Kingdom). [8-3H]Hypoxanthine was from Amersham Life Sciences (Buckinghamshire, United Kingdom). A23187 was from Calbiochem-Novabiochem (Nottingham, United Kingdom). Phosphate-buffered saline (PBS) was from Life Technologies (Paisley, United Kingdom). CaCl2, MgCl2, NaCl, and KCl were from FSA Laboratory Supplies (Loughborough, United Kingdom). Arabinogalactan was from Larex (White Bear Lake, MN). Percoll was from Amersham Pharmacia Biotech (Uppsala, Sweden).
Effect of RBC volume on parasite count at 24 hours after initiation of culture
Initial [K+], mM . | Parasite count at 24 h, % . | . | . | ||
|---|---|---|---|---|---|
| . | A4 . | W2 . | FCR3 . | ||
| Untreated | 15.4 | 9.0 | 10.0 | ||
| 137 K+ | 15.4 | 6.8 | 11.4 | ||
| 77-80 K+ | 8.9 | 4.4 | 9.6 | ||
| 22-45 K+ | 1.5 | 1.5 | 4.8 | ||
| 2-3 K+ | 2.8 | 0.8 | 0.8 | ||
Initial [K+], mM . | Parasite count at 24 h, % . | . | . | ||
|---|---|---|---|---|---|
| . | A4 . | W2 . | FCR3 . | ||
| Untreated | 15.4 | 9.0 | 10.0 | ||
| 137 K+ | 15.4 | 6.8 | 11.4 | ||
| 77-80 K+ | 8.9 | 4.4 | 9.6 | ||
| 22-45 K+ | 1.5 | 1.5 | 4.8 | ||
| 2-3 K+ | 2.8 | 0.8 | 0.8 | ||
Experimental design
The effects of the RBC hydration state on parasite invasion and growth were investigated in 2 separate studies using different experimental protocols and various P falciparum strains. In the first study (study I) cultures were sustained with fresh, normal RBCs whose volume was altered isotonically by a reversible increase in K+ permeability. In the second study (study II), normal RBCs and RBCs with different genetic abnormalities were density-fractionated and the different density fractions tested for their ability to sustain P falciparum cultures. We describe each protocol in detail (see “Study I” and “Study II”). Approval for these studies was obtained from the Institutional Review Boards, or the equivalent ethical bodies, of the University of Cambridge, Cambridge, United Kingdom; the Lawrence Berkeley National Laboratory, Berkeley, CA; and the Hebrew University of Jerusalem, Jerusalem, Israel.
Cultures
Five different strains of P falciparum (A4,15 W2, and FCR3 in study I; FCR3K+ and ITgC32p21 in study II) were cultured in human erythrocytes by standard methods16,17 under a low-oxygen atmosphere. The culture medium was RPMI 1640, supplemented with 40 mM HEPES, 25 mg/L gentamicin sulfate, 10 mM d-glucose, 2 mM glutamine, and either 8.5% (vol/vol) serum (strains FCR3K+, ITgC32p21, and A4 clone) or 8% heat-inactivated plasma (strains W2 and FCR3).
Study I
The approach was to assess P falciparum invasion and growth in standard malaria cultures using normal RBCs with altered volumes. A procedure was required that would enable resetting the RBC volume to any desirable level ensuring its constancy in culture. Volume changes induced by hypertonic or hypotonic conditions were thus excluded because the high water permeability of RBCs would rapidly re-equilibrate the RBCs in the isotonic culture medium thus reversing the induced volume changes. It was clear that the volume changes had to be implemented in isotonic conditions. This was done by inducing a reversible permeability change in the RBC plasma membrane allowing a reduction or an increase in the total content of osmotically active solutes within the RBCs. The most abundant osmolytes in normal RBCs are K+ and Cl- and there is also a ready procedure to induce a reversible increase in the K+ permeability.
The design of the experimental protocol followed leads from earlier work, as described next. Of the 2 tools available to selectively permeabilize RBCs to K+, valinomycin and increased intracellular Ca2+ (operating via Gardos channels10 ), only Ca2+ loading can be reversed. Using Ca2+ ionophores (eg, A2318718,19 ) it is possible to uniformly load all RBCs in a given suspension with enough Ca2+ to maximally activate the Gardos channels in all the cells.20,21 In the Gardos-activated state, RBCs readily equilibrate with the extracellular K+ concentration and adjust their volumes accordingly, swelling in media containing over 90 mM K+ and shrinking in media with lower K+ concentrations. Addition of excess EGTA over external Ca2+ to the RBC suspension rapidly extracts the Ca2+ load from the cells and shuts off the Gardos channels, restoring the original low K+ permeability of the cells. Subsequent RBC washes with albumin-containing medium removes the ionophore from the RBC plasma membrane and reverses Ca2+ permeabilization, leaving the cells largely unable to recover their original volume when incubated in the isotonic culture media. An important caveat applies to the duration of the Ca2+ load required for K+ equilibration. Ca2+-loading activates the PMCA, triggering adenosine triphosphate (ATP) hydrolysis at rates exceeding the normal rate of glycolysis in most cells.20,22 The combined activities of the PMCA adenosine triphosphatase (ATPase), adenylate kinase, and adenosine monophosphate (AMP) deaminase convert ATP to inosine monophosphate (IMP) leading to irreversible ATP depletion.23-25 One way to minimize such effect is to reduce the Ca2+-loading period to a minimum, but a compromise is required to allow for K+ equilibration. The rate of K+ equilibration in K+-permeabilized RBCs is rate-limited by the Cl- permeability.26,27 Isotonic replacement of no more than 10 mM Cl- by an equal concentration of SCN- speeds up KCl equilibration approximately 50-fold, allowing the Ca2+-loading, K+-equilibration period to be reduced to 10 minutes or less, with minimal effects on cell metabolism.9,21
Preparation of cells with different volumes
RBCs were obtained from healthy volunteers by venipuncture into heparinized syringes after informed, written consent was obtained according to the Declaration of Helsinki. Fresh RBCs were washed twice by centrifugation and resuspension in over 10 volumes of wash medium W with 0.1 mM EGTA, and thrice more with solution W. The buffy coat was removed after each wash. After the last wash the cells were suspended at 5% hematocrit in isotonic solutions with the final K+ concentrations indicated in the figures (see “Chemicals and solutions”), supplemented with CaCl2, and incubated at 37°C under magnetic stirring. After about 10 minutes, to allow for temperature equilibration, the ionophore A23187 was added (t = 0) to give a final concentration of 10 μM in each suspension (except in the untreated control groups) and the incubation continued for a further 10 minutes to allow for full K+ equilibration. At t = 10 minutes, EGTA was added to each suspension to extract the Ca2+ from the cells and deactivate the Gardos channels. About 5 minutes later, each suspension was transferred into centrifuge tubes containing 9 volumes of ice-cold wash medium W supplemented with 0.1 mM EGTA and 2% defatted bovine serum albumin, to initiate the extraction of the ionophore A23187. The suspensions were centrifuged at 800g for 5 minutes, the cells were washed twice more by centrifugation and resuspension in the same medium at a volume-cell ratio of about 100:1, and thrice more in phenol red-free RPMI 1640 to wash away the albumin. The use of phenol red-free RPMI was necessary to prevent interference with the measurements of Hb concentrations described. RBCs that were not treated with ionophore were used for control cultures. The mean RBC volume and volume distribution of untreated RBCs and of RBCs equilibrated at the different external K+ concentrations were assessed by measuring the osmotic fragility profiles of each suspension, as previously described.28-30 Briefly, aliquots of the cells from each group were resuspended at 5% hematocrit in phenol red-free RPMI 1640 and assayed for osmotic fragility on microwell plates. The Hb concentration was determined in lysates by light absorption at 415 nm using a Multiscan Plate Reader (Molecular Devices, Wokingham, United Kingdom). In 2 experiments, the time-dependent shifts in osmotic fragility curves of profoundly dehydrated RBCs were followed over a period of time in parallel with measurements of cell density distribution by separation through phthalate oils,31 to ascertain whether the observed shifts in the osmotic fragility curves represented rehydration of the cells or loss of membrane area due to microvesiculation.
Assessment of P falciparum invasion and growth in cultures with RBCs of different volumes
Invasion of P falciparum was assessed in cultures by microscopic inspection of Giemsa-stained thin blood smears and parasite count. At least 2000 RBCs were counted and the parasitemia was reported as the percent of parasitized erythrocytes. P falciparum growth was assessed by measuring the incorporation of the radiolabeled nucleic acid precursor [3H]hypoxanthine.32 Three different P falciparum strains, A4, W2, and FCR3, were used for this set of experiments.
Parasites were stage-synchronized by Percoll gradient purification.33 The recovered schizont-containing RBCs were used to start the different cultures at 1% hematocrit and 3% to 4% parasitemia. Fresh, volume-altered RBCs were used in 4 of the 5 cultures; in the fifth, untreated RBCs (control) were used. The inoculum contained a negligible amount of uninfected cells so that merozoite invasion was almost exclusively into the freshly added RBCs. After 24 hours, culture samples for Giemsa-stained smears were obtained from each culture condition to assess invasion by means of parasite count and to evaluate parasite morphology. Immediately thereafter the parasites were synchronized at the ring stage with d-sorbitol34 to get rid of all older stages, the cells washed in RPMI 1640, resuspended in culture medium at about 2% hematocrit and incubated with [3H]hypoxanthine (5-20 μCi/mL, [0.185-0.740 MBq] final concentration) at 37°C and low-oxygen atmosphere for about 4 to 24 hours to assess parasite growth. After the incubation period the cells were harvested and [3H]hypoxanthine incorporation was measured as described.
In the experiments with strains W2 and FCR3, the cultures were incubated in triplicate with [3H]hypoxanthine for 5 hours before cell harvesting for radioactive counting. In the experiments with strain A4, cultures were followed for 4 hours and 24 hours before cell harvesting for radioactive counting.
Study II
P falciparum (strains FCR3K+ or ITgC32p21) invasion was assessed in density-separated fractions of normal RBCs and of RBCs with different genetic abnormalities. Blood samples were obtained by venipuncture in citrate medium, from healthy volunteers and from individuals with various RBC disorders, after informed written consent was obtained. The previously established diagnoses of the various RBC disorders were reconfirmed on the basis of RBC analysis, Hb electrophoresis, and biochemical analysis. The RBC congenital abnormalities included (number indicated in parenthesis): α-thalassemia trait (1), Hb H (1), β-thalassemia trait (4), hereditary elliptocytosis (4), xerocytosis (2), Hb SC (2), and Hb CC (1). Blood samples were kept at 4°C immediately after collection and used within 30 hours. None of the individuals had received blood transfusions for at least 12 weeks prior to sample collection.
RBCs were separated in different fractions using a discontinuous arabinogalactan gradient.35 RBCs were washed thrice by centrifugation and resuspension in PBS and the buffy coat removed each time. After the washes, the RBCs were resuspended in the same solution at 40% hematocrit and layered above the arabinogalactan gradient.35-39 The gradient consisted of several fractions spanning a range of 19% to 31% arabinogalactan in PBS (wt/vol) in 2% increments, all strictly isotonic, rendering the densities and mean cell Hb concentrations reported in Table 1 and Figure 1. The tube in which the gradient was placed was spun in a SW 27 swing-out rotor (Beckman Instruments, Fullerton, CA) at 20 000 rpm for 45 minutes at 15°C. RBCs from each density fraction were then carefully harvested, washed twice in PBS, washed once in RPMI 1640, resuspended in culture medium, and used for invasion assays.
Relation between the concentrations of arabinogalactan used for density fractionation of RBCs, their corresponding density, and MCHC measured in normal RBCs harvested from the different density layers
Percent arabinogalactan, wt/vol . | Density, g/mL . | MCHC, g/dL . |
|---|---|---|
| 19 | 1.079 | 27.6 |
| 21 | 1.088 | 30.8 |
| 23 | 1.096 | 34.4 |
| 25 | 1.106 | 36.9 |
| 27 | 1.115 | 39.1 |
| 29 | 1.124 | 42.6 |
| 31 | 1.134 | 46.2 |
Percent arabinogalactan, wt/vol . | Density, g/mL . | MCHC, g/dL . |
|---|---|---|
| 19 | 1.079 | 27.6 |
| 21 | 1.088 | 30.8 |
| 23 | 1.096 | 34.4 |
| 25 | 1.106 | 36.9 |
| 27 | 1.115 | 39.1 |
| 29 | 1.124 | 42.6 |
| 31 | 1.134 | 46.2 |
Parasites were stage synchronized by Percoll gradient purification. The gradient consisted of 3 layers of Percoll in PBS (from top to bottom: 40% [2 mL], 70% [3 mL], and 90% [4 mL] Percoll) placed in centrifuge tubes. RBCs from the parasitized culture were spun down, resuspended in RPMI 1640 (1:1 vol/vol) and carefully placed on top of the gradient. The tubes were centrifuged at 36 000 g for 20 minutes at 20°C. The cells at the 40% to 70% interface, containing mostly schizonts were collected, washed twice in RPMI, resuspended in culture medium, and used as inoculum for the different cultures. The cultures were started at 1% to 2% hematocrit and parasitemias between 2% and 5% for the different experiments. Untreated normal RBCs or untreated RBCs with the different congenital abnormalities were always used as controls. After 38 to 40 hours of incubation, culture samples for measuring parasitemias on Giemsa-stained blood smears were obtained from each condition. Morphologically normal and abnormal parasites were included in the measurements. Parasite counts were reported as the percent of parasitized erythrocytes. At least 1000 RBCs per slide were counted. A minimum of 3 slides was examined for each condition.
Results
Study I
Volume stability of volume-modified RBCs. The osmotic fragility distribution of populations of normal RBCs with and without modified K+ contents is shown in Figure 2A. The results show both left and right shifts in the osmotic fragility curves of RBCs with altered K+ content relative to that of untreated controls, reflecting dehydration (left shift) of RBCs equilibrated at low extracellular K+ concentrations (2, 22, and 77 mM), and swelling (right shift) of cells equilibrated at 137 mM extracellular K+. The volume of each RBC is determined by its osmolyte and Hb contents.29,41 The method applied here to alter the RBC volume affected primarily the osmolyte content of the cells, increasing or decreasing their K+ and Cl- contents. Thus, the shifts in the osmotic fragility curves shown in Figure 2A reflect the volume changes associated with either a decrease or an increase in RBC K+ and Cl- contents. As originally pointed out by Ponder,40 hemolysis curves represent the integrals of normal (Gaussian) distributions. Thus, the conservation of the hemolysis curve profile of volume-altered RBCs relative to that of controls indicates that the original Gaussian parameters of the distribution were largely preserved in both dehydrated and hydrated states.
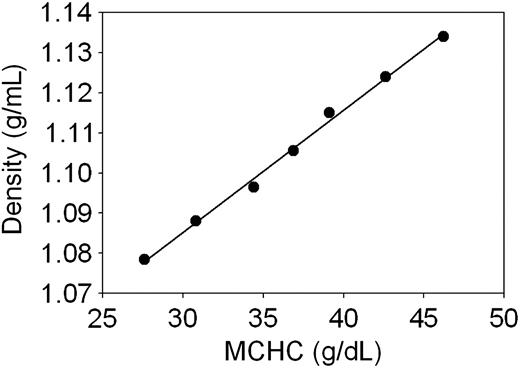
Linear regression fit of the correlation between cell density and MCHC in normal human RBCs. The points represent data from Table 1, column 2 plotted against column 3. The regression coefficient was r2 = 0.996. The following equation reports the parameters of the fit and can be applied to estimate intermediate and extrapolated data within and outside the measured range: density = 0.00304*MCHC + 0.994.
Linear regression fit of the correlation between cell density and MCHC in normal human RBCs. The points represent data from Table 1, column 2 plotted against column 3. The regression coefficient was r2 = 0.996. The following equation reports the parameters of the fit and can be applied to estimate intermediate and extrapolated data within and outside the measured range: density = 0.00304*MCHC + 0.994.
The osmotic fragility curves in Figure 2A reflect the condition of RBCs at the time the cultures were initiated. The volume distributions reflected in these curves remained stable in culture in all conditions except in the most dehydrated cells. Figure 2B shows how the volume distribution changed with time in this particular group. There was a slow, spontaneous shift to the right with considerable broadening of the osmotic fragility curve at 4 and 20 hours. Measurements of density distribution changes in the most dehydrated cells (not shown), run in parallel with the osmotic fragility measurements, revealed slow, uneven cell density shifts toward lower densities. This indicates progressive rehydration of a substantial subpopulation of initially profoundly dehydrated RBCs. Microscopic observation of fresh, unfixed culture samples of markedly dehydrated RBCs showed a progressive development of cell volume heterogeneity associated with a gradual appearance of smooth, flat, discoid RBC shapes, some with incipient or well-developed biconcave disk appearance of normal diameter, suggesting rehydration to normal sizes rather than “smoothing” to microcytic forms by exovesiculation in the initially crenated cells (Figure 3). The mechanism of this spontaneous and uneven rehydration response is the subject of a separate investigation, but the relevant point here is that over the period in which merozoites were released in the culture conditions of this study, a fraction of initially profoundly dehydrated RBCs became partially rehydrated.
Osmotic fragility curves of K+-equilibrated RBCs. (A) The numbers next to the curves indicate the initial [K+], in mM, in the extracellular media used for K+ equilibration, as described in “Materials and methods” for study I. Open and filled paired symbols report duplicate measurements for each condition. Left shifts relative to the untreated controls indicate RBC dehydration; right shifts indicate RBC swelling. The curves represent integrals of near Gaussian distributions.29,40 Therefore, the approximate parallelism in the observed shifts indicates that the population variation in RBC volumes did not change much in the different hydration states relative to that in the untreated controls, despite the large changes in mean cell volume. (B). Time-dependent shifts in the osmotic fragility curves of markedly dehydrated RBCs (K+ equilibrated in 3 mM [K+]). The curve at 10 minutes approximates the initial osmotic fragility pattern of the dehydrated RBCs at the start of culture. With time (4 hours and 20 hours), there is a right shift in the osmotic fragility curves, reflecting spontaneous rehydration. The progressive decrease in slope indicates increasing population variation of RBC volumes.
Osmotic fragility curves of K+-equilibrated RBCs. (A) The numbers next to the curves indicate the initial [K+], in mM, in the extracellular media used for K+ equilibration, as described in “Materials and methods” for study I. Open and filled paired symbols report duplicate measurements for each condition. Left shifts relative to the untreated controls indicate RBC dehydration; right shifts indicate RBC swelling. The curves represent integrals of near Gaussian distributions.29,40 Therefore, the approximate parallelism in the observed shifts indicates that the population variation in RBC volumes did not change much in the different hydration states relative to that in the untreated controls, despite the large changes in mean cell volume. (B). Time-dependent shifts in the osmotic fragility curves of markedly dehydrated RBCs (K+ equilibrated in 3 mM [K+]). The curve at 10 minutes approximates the initial osmotic fragility pattern of the dehydrated RBCs at the start of culture. With time (4 hours and 20 hours), there is a right shift in the osmotic fragility curves, reflecting spontaneous rehydration. The progressive decrease in slope indicates increasing population variation of RBC volumes.
Morphologic appearance of fresh, unfixed samples of initially profoundly dehydrated RBCs at different times of incubation in culture media. RBCs in culture medium were observed under phase contrast at × 630 (Zeiss photomicroscope III RS; Zeiss Planapo objective lens, ×63 magnification, 1.4 numerical aperture). Photographs were taken with a JVC color video camera, TK-C1381. Note the gradual appearance of smooth, flattened discs, some with typical biconcave shape, indicating spontaneous rehydration of RBCs. This explains the developing heterogeneity apparent in the osmotic fragility curves of Figure 2B.
Morphologic appearance of fresh, unfixed samples of initially profoundly dehydrated RBCs at different times of incubation in culture media. RBCs in culture medium were observed under phase contrast at × 630 (Zeiss photomicroscope III RS; Zeiss Planapo objective lens, ×63 magnification, 1.4 numerical aperture). Photographs were taken with a JVC color video camera, TK-C1381. Note the gradual appearance of smooth, flattened discs, some with typical biconcave shape, indicating spontaneous rehydration of RBCs. This explains the developing heterogeneity apparent in the osmotic fragility curves of Figure 2B.
Comparative parasitemias and [3H]hypoxanthine incorporation patterns in cultures sustained with volume-modified RBCs. The results of 3 similar experiments, each with a different P falciparum strain, are shown in Table 2. Parasite counts, as a measure of invasion, were estimated in cultures sustained with untreated RBCs (controls) or with RBCs subjected to K+ equilibration in media containing different K+ concentrations. Parasite counts in cultures sustained with untreated RBCs and those in cultures sustained with swollen RBCs (equilibrated in 137 mM [K+]) were comparable, indicating that transient exposure of RBCs to ionophore A23187 and high-[Ca2+]i did not have any detectable effects on invasion efficiency per se. Cultures sustained with RBCs equilibrated in lower K+ concentrations showed a progressive marked decline in parasitemia. There was near-complete inhibition of invasion in the profoundly dehydrated RBCs. Microscopic observation of Giemsa-stained blood smears of this culture showed merozoites adhered to the RBC surface but not internalized. The minute fraction of RBCs with ring-stage parasites associated with this cell fraction was seen only within rehydrated RBCs. The likely reason why only a small fraction of rehydrated cells was invaded in this group is because by the time the dehydrated cells rehydrate, no more schizonts are available in the synchronized culture to provide new merozoites for invasion. The microscopic appearance of cultures sustained with untreated RBCs and of those sustained with hydrated RBCs (equilibrated in 137 mM K+) was indistinguishable, suggesting once again that transient ionophore exposure and Ca2+ loading of RBCs had no effect on invasion efficiency and parasite growth.
[3H]hypoxanthine incorporation in P falciparum cultures sustained with RBCs in different hydration states. Following parasite invasion in cultures sustained with RBCs of different volumes, parasites were ring-stage synchronized and incubated with [3H]hypoxanthine for 24 hours. After this period, parasite growth was assessed by measuring [3H]hypoxanthine incorporation, as described in “Materials and methods.” The columns and error bars report the means and SDs of quadruplicate measurements expressed as a percent of the incorporation in untreated controls (right column).
[3H]hypoxanthine incorporation in P falciparum cultures sustained with RBCs in different hydration states. Following parasite invasion in cultures sustained with RBCs of different volumes, parasites were ring-stage synchronized and incubated with [3H]hypoxanthine for 24 hours. After this period, parasite growth was assessed by measuring [3H]hypoxanthine incorporation, as described in “Materials and methods.” The columns and error bars report the means and SDs of quadruplicate measurements expressed as a percent of the incorporation in untreated controls (right column).
Parasite growth was assessed by measuring [3H]hypoxanthine incorporation in P falciparum cultures sustained with volume-modified RBCs and with untreated RBCs. The results of one experiment, representative of 4 with strains A4, W2, and FCR3 are shown in Figure 4. After parasite invasion in cultures sustained with RBCs of different volumes, parasites were synchronized to the ring stage and incubated with [3H]hypoxanthine for 24 hours before measuring [3H]hypoxanthine incorporation. The results with untreated RBCs where comparable to those with RBCs equilibrated in solutions containing [K+] above 77 mM (Figure 4). The increased uptake shown here at [K+] = 137 mM was not reproduced in the other 3 experiments, where there was no significant difference between untreated and hydrated RBCs. The similarity of uptakes in untreated and hydrated RBCs shows again that transient ionophore exposure and Ca2+ loading per se had no detectable effects on parasite growth or infectivity. A consistent result in all strains was the significant decline in [3H]hypoxanthine incorporation with RBC dehydration, when the inoculum was applied to RBCs equilibrated in media with initial [K+] below 45 mM.
Figure 5 shows the results of an experiment where [3H]hypoxanthine incorporation was measured at 4 hours (A) and 24 hours (B) in culture (note ordinate scale differences). Incorporation of [3H]hypoxanthine at 4 hours measures invasion efficiency, whereas at 24 hours it reflects both invasion and parasite growth. The ratio (24 hours-4 hours)/4 hours, plotted in panel C, subtracts the invasion component and allows a better comparison of parasite growth and development in the different conditions. Panel C in Figure 5 shows that whereas the rate of parasite growth is comparable between untreated controls, mildly dehydrated (77 K) and mildly overhydrated (137 K) RBCs, it is substantially inhibited in dehydrated RBCs. It remains to be clarified whether this inhibition of parasite growth reflects a uniform pattern in all the rehydrated cells that were infected or an heterogeneous pattern with some parasites developing normally and others severely impaired. In cultures initiated with profoundly dehydrated RBCs and followed past 60 hours, with medium changes but no further RBC additions, Giemsa-stained smears showed that at least some of the parasites that had invaded rehydrated RBCs were able to develop through normal-appearance trophozoite and schizont stages and reinfect other rehydrated cells. This result supports the heterogeneous alternative and also indicates that, as with invasion, normal growth conditions in the rehydrated RBCs are not irreversibly lost.
Study II
Figure 6 reports the results of experiments with normal RBCs from 4 different donors, density-fractionated on discontinuous arabinogalactan gradients, as described in “Materials and methods.” Cultures were started with stage-synchronized schizonts, and parasite counts were measured after 38 hours. There was a consistent pattern of gradual reduction in parasite counts with increased density of the RBCs. There is thus a definite preference for parasite invasion in the lesser dense RBCs.
[3H]hypoxanthine incorporation after 4 hours and 24 hours of incubation of P falciparum cultures sustained with RBCs in different hydration states. The experimental protocol was similar to that indicated in Figure 4 except that [3H]hypoxanthine incorporation was measured at 4 hours (A), representing invasion efficiency, and 24 hours (B), reflecting invasion and parasite growth. Note the orders of magnitude difference in the ordinate scales between panels A and B. Panel C reports the [3H]hypoxanthine incorporation ratio (24 hours-4 hours)/4 hours, estimated from randomly paired samples. This ratio is assumed to reflect mainly parasite growth rate because the extent of [3H]hypoxanthine incorporation attributed to invasion at 4 hours is subtracted in the calculations. The right columns in each panel report [3H]hypoxanthine incorporation in untreated controls. Columns and error bars represent means and SDs of triplicate measurements.
[3H]hypoxanthine incorporation after 4 hours and 24 hours of incubation of P falciparum cultures sustained with RBCs in different hydration states. The experimental protocol was similar to that indicated in Figure 4 except that [3H]hypoxanthine incorporation was measured at 4 hours (A), representing invasion efficiency, and 24 hours (B), reflecting invasion and parasite growth. Note the orders of magnitude difference in the ordinate scales between panels A and B. Panel C reports the [3H]hypoxanthine incorporation ratio (24 hours-4 hours)/4 hours, estimated from randomly paired samples. This ratio is assumed to reflect mainly parasite growth rate because the extent of [3H]hypoxanthine incorporation attributed to invasion at 4 hours is subtracted in the calculations. The right columns in each panel report [3H]hypoxanthine incorporation in untreated controls. Columns and error bars represent means and SDs of triplicate measurements.
Figure 7 shows the results of experiments, similar to those reported in Figure 6, but using RBCs with various RBC abnormalities, encompassing RBCs with abnormal hemoglobins or plasma membrane defects (α-thalassemia trait, Hb H and β-thalassemia trait, hereditary elliptocytosis, and xerocytosis). RBCs from each donor were density-separated in different fractions and each fraction used to sustain P falciparum cultures. Parasite invasion was assessed after 38 hours of incubation in standard culture conditions. The results reported for β-thalassemia trait are representative of 4 experiments using RBCs from 4 different donors with this abnormality; the results reported for hereditary elliptocytosis are representative of 4 different experiments, and those for xerocytosis are representative of 2 different experiments. Irrespective of the RBC pathology or density range, the consistent pattern observed in all experiments, including one experiment with Hb CC and 2 with Hb SC RBCs (not shown), was that parasite count, representing parasite invasion, declined with increased RBC density, particularly so in the highly dehydrated xerocytic RBCs.42 The extent of the decline varied widely but the pattern of monotonic decline with density was always present in all abnormal RBCs studied. These findings attest to an important role for the state of RBC hydration in modulating parasite invasion even in the face of various RBC abnormalities.
P falciparum invasion efficient in normal RBCs of different densities. Normal RBCs from 4 different donors were harvested from the indicated arabinogalactan concentration ranges and used to sustain P falciparum cultures as described in “Materials and methods.” Parasite counts were assessed on Giemsa-stained blood smears after 38 hours of incubation of the culture. At least 1000 RBCs were counted per slide on 3 different slides. “All” indicates parasite counts in the original, unfractionated RBC samples. The columns and error bars report the means and SDs of the triplicate counts. Labels 1-4 in quadrants indicate a different donor.
P falciparum invasion efficient in normal RBCs of different densities. Normal RBCs from 4 different donors were harvested from the indicated arabinogalactan concentration ranges and used to sustain P falciparum cultures as described in “Materials and methods.” Parasite counts were assessed on Giemsa-stained blood smears after 38 hours of incubation of the culture. At least 1000 RBCs were counted per slide on 3 different slides. “All” indicates parasite counts in the original, unfractionated RBC samples. The columns and error bars report the means and SDs of the triplicate counts. Labels 1-4 in quadrants indicate a different donor.
Discussion
Protection against falciparum malaria is closely linked to the high prevalence of inherited RBC abnormalities in malaria endemic regions.43,44 These abnormalities may affect the structure of hemoglobin,45,46 the RBC membrane,47,48 or RBC metabolism,49-51 generating a large diversity of pathologies affecting millions of people worldwide. The most widely distributed of these pathologies are sickle cell anemia, thalassemias,52-54 ovalocytosis,48,55 hereditary spherocytosis,56 and glucose-6-phosphate dehydrogenase (G6PD) deficiency.51 From the little we know about the mechanisms of protection a bewildering variety of strategies seems to apply.45,57 In thalassemias, it is still debated whether protection arises from immune vulnerability or from excessive oxidative damage of RBCs with mature parasite stages.58-61 In ovalocytosis and hereditary spherocytosis, protection is clearly linked to specific cytoskeletal abnormalities causing increased RBC rigidity and impaired invasion, although the precise mechanism remains unknown62 ; hereditary spherocytosis is rarely found in tropical countries. In G6PD deficiency, RBCs with ring-stage parasites are selectively phagocytosed by human adherent monocytes, probably due to RBC membrane damage resulting from impaired antioxidant defenses.58 In sickle cell Hb carriers, both heterozygous and homozygous individuals are protected by an unknown mechanism.63,64 Although the debilitated condition of SS homozygous patients increases the lethality of malaria infection, this is not necessarily through cerebral malaria. Thus, only heterozygous individuals benefit in a selectively valid sense.65-68 Whatever the protective mechanism against malaria, the development of dangerously high parasitemias in any of these RBC pathologies is reduced, promoting the survival of abnormal gene carriers in the population.
The results presented here document reduced P falciparum invasion in experimentally dehydrated normal RBCs and in dense RBC fractions from healthy subjects and from individuals with a variety of inherited RBC abnormalities. Parasite invasion and growth were studied in P falciparum cultures using 5 different parasite strains. Invariably, artificially dehydrated RBCs, and naturally occurring dense RBCs isolated from fresh blood samples, showed reduced parasite invasion and growth. These findings suggest that in a number of inherited RBC pathologies, the presence of dense RBCs adds protection against severe malaria by reducing the competence of the target RBCs to sustain normal merozoite invasion in the microcirculation, where the RBCs with mature parasites are sequestered.
P falciparum invasion efficiency in density-separated RBCs with various congenital abnormalities. RBCs from 6 different donors with the indicated pathologies were harvested from the arabinogalactan concentration ranges shown on the abscissa and used to sustain P falciparum cultures in conditions similar to those indicated in Figure 6. “All” indicates parasite counts in the original, unfractionated RBC samples. Parasite counts were assessed after 38 hours of incubation on Giemsa-stained blood smears. At least 1000 RBCs were counted per slide on 3 different slides. The columns and error bars report the means and SDs of these triplicate counts.
P falciparum invasion efficiency in density-separated RBCs with various congenital abnormalities. RBCs from 6 different donors with the indicated pathologies were harvested from the arabinogalactan concentration ranges shown on the abscissa and used to sustain P falciparum cultures in conditions similar to those indicated in Figure 6. “All” indicates parasite counts in the original, unfractionated RBC samples. Parasite counts were assessed after 38 hours of incubation on Giemsa-stained blood smears. At least 1000 RBCs were counted per slide on 3 different slides. The columns and error bars report the means and SDs of these triplicate counts.
The procedure used to alter the volume of normal RBCs in this study is known to induce a variety of structural and metabolic changes such as cross-linking of cytoskeletal proteins,69 and partial but irreversible ATP depletion.20,22-24 RBCs were transiently exposed to ionophore treatment, high Ca2+ loads, and prolonged washes in different media. Yet these maneuvers did not affect their ability to sustain normal P falciparum invasion and growth provided the volume of the RBCs remained near normal levels. The observed results with dehydrated RBCs may thus safely be attributed to the RBC hydration state and not to any side effects of the applied treatments.
Although the mechanism of the effect of RBC hydration state on falciparum invasion remains unexplained, the results provide some important insights on its main characteristics. Dehydration of normal RBCs, as performed here, affected all RBCs regardless of age or density. In the profoundly dehydrated RBCs, parasite invasion was fully blocked. Because RBCs of all ages participated in preventing invasion, this effect must be attributed to their dehydrated state, not their age. Because of the well-known relationship between density and cell age in normal human RBCs,70 reduced invasion in the denser cells may be interpreted as an age-related phenomenon, as was done in the past, rather than as a volume or density effect.71-73 Cyclic AMP-dependent kinase has been shown to be indispensable for invasion,74 suggesting that aged red cells may be less susceptible to invasion. However, to the extent that the suggested age relation was based on density separation of RBCs, it may need re-evaluation in the light of the present results, to establish whether cell age, in addition to the hydration state of the RBC participates in the reduced infectivity of the denser cells.
The reversible nature of the invasion block in profoundly dehydrated RBCs restricts the range of plausible explanations of the RBC hydration-state effects by excluding irreversible membrane and cytoskeletal changes associated with volume or density changes. As shown in Figure 3, Ca2+-dehydrated RBCs appear uniformly crenated at the start of the cultures. In time, increasing fractions of cells rehydrate, regaining a smooth discoid shape and recovering their ability to sustain parasite invasion. Lesser dehydrated cells show fewer crenations and appear more like flattened discs in culture, yet their infectivity and ability to sustain parasite growth is still substantially reduced compared to untreated RBCs (Table 2; Figures 4, 5). Thus, geometrical hindrances to merozoite attachment may not be particularly relevant. The dehydration effects on the RBC are likely to be more subtle and complex, related to the myriad of membrane-cytoskeletal rearrangements during merozoite attachment and internalization.3
Additional factors to be considered in relation to the reduced infectivity of dense RBCs are molecular crowding, changes in intracellular pH, and the effects of the hydration state on the mechanical properties of the RBC membrane. Increased density is equivalent to increased Hb concentration (Table 1). Hb binding to band 3 is oxygen sensitive75 and the extent of binding may be influenced by the Hb concentration, thus indirectly interfering with the membrane-cytoskeletal changes following merozoite attachment.
Dehydration of RBCs by KCl loss reduces the intracellular Cl- concentration.14,76 The operation of the anion exchanger in parallel with the CO2 shunt, known as the Jacobs-Stewart mechanism,77,78 maintains an equilibrium in which the ratio of intracellular-extracellular proton concentrations equals the extracellular-intracellular Cl- concentration ratio. Because of the reduced intracellular Cl- concentration in the dehydrated cells, their extracellular-to-intracellular Cl- concentration ratio will be increased and so will the intracellular-to-extracellular proton concentration ratio. Consequently, the intracellular pH of RBCs suspended in well-buffered, high-Cl- cultured media will be lower the more dehydrated the condition of the RBCs, and this extra intracellular acidity may participate in reduced parasite invasion. Rehydrating RBCs, on the other hand, can only increase their volume by the isotonic gain of NaCl, driven by the inward Na+ gradient. The resulting increase in intracellular Cl- concentration reverses the acidification trend during dehydration, consistent with a role of cell pH in the restoration of infectivity.
The mechanical properties of the RBC membrane have been shown to be regulated primarily by the spectrin-actin-based cytoskeletal network through lateral interactions among skeletal proteins and vertical interactions between skeletal components and transmembrane proteins in the bilayer.79-81 Such interactions, which are thought to be instrumental in the invasion of the RBC by the malaria parasite2 are likely to be affected by the hydration state of the RBC.
Much future work will be required to solve the mechanism of reduced infectivity of the denser RBCs. Further speculation at this stage is not warranted.
Prepublished online as Blood First Edition Paper, February 22, 2005; DOI 10.1182/blood-2004-12-4948.
Supported by grants 061269 and 059725 from the Wellcome Trust (United Kingdom), and grant DK 32094 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Lynn Macdonald for excellent technical assistance.

![Figure 2. Osmotic fragility curves of K+-equilibrated RBCs. (A) The numbers next to the curves indicate the initial [K+], in mM, in the extracellular media used for K+ equilibration, as described in “Materials and methods” for study I. Open and filled paired symbols report duplicate measurements for each condition. Left shifts relative to the untreated controls indicate RBC dehydration; right shifts indicate RBC swelling. The curves represent integrals of near Gaussian distributions.29,40 Therefore, the approximate parallelism in the observed shifts indicates that the population variation in RBC volumes did not change much in the different hydration states relative to that in the untreated controls, despite the large changes in mean cell volume. (B). Time-dependent shifts in the osmotic fragility curves of markedly dehydrated RBCs (K+ equilibrated in 3 mM [K+]). The curve at 10 minutes approximates the initial osmotic fragility pattern of the dehydrated RBCs at the start of culture. With time (4 hours and 20 hours), there is a right shift in the osmotic fragility curves, reflecting spontaneous rehydration. The progressive decrease in slope indicates increasing population variation of RBC volumes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/12/10.1182_blood-2004-12-4948/5/m_zh80120580060002.jpeg?Expires=1767701832&Signature=wGsfLKLaHKvj-coioIPW4WMutHGe2r1JW6WfB8C6wi98vcoZASoqvklZLO57kHhDL8UTkLinljbs1sWjloRiNyYa6W5gckLhBYjrAeqZzzFKGksn3G~KfPuVybmFvqlPRe844XCiRGXRUTA1xJSe8D0VRTjCpNkixVp-kRTuY9M0C1d98PH0eIYz6ZRNKoo8b1MSpFDh-m5HpkPIV9rWUQHL-rFv44LtCkH9GGATcEIFbk~fcUDfLaC6SB~UB8Ve~BVgOV-4xFcBk2QP-VvlqRnPLlb~KNLfx3vTnZ4m~V79vXyBADqGLwc2mOThohpJIy8Nhpd0zYsXBmZ-P5B0uw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. [3H]hypoxanthine incorporation in P falciparum cultures sustained with RBCs in different hydration states. Following parasite invasion in cultures sustained with RBCs of different volumes, parasites were ring-stage synchronized and incubated with [3H]hypoxanthine for 24 hours. After this period, parasite growth was assessed by measuring [3H]hypoxanthine incorporation, as described in “Materials and methods.” The columns and error bars report the means and SDs of quadruplicate measurements expressed as a percent of the incorporation in untreated controls (right column).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/12/10.1182_blood-2004-12-4948/5/m_zh80120580060004.jpeg?Expires=1767701832&Signature=hnUisZaV9InsmPUSg65Nl7kJCHwLvLIWPjD1HqG-vvE8kvz5sedfx2RqY3ey4G2~bNrbE7XWsIge1BD~39qErZqLQNKFz3a0xuqPJwLZ9Ju6ZgtBvlkE17C0VoxBY4i29kE5Kd2sLs~ecG1EZDCijbkhQB-j6a2muEmkfJ0-H5u~Rpd981vdIE9HtSE87Kbt0bgCScwiySEcLhQxtTWxlVvZcFvV7lnv6WTSGZUOB~ltVJYqwIchGHENuz58bfNIu6rU820hCIeFVXCpkvC8YV5t2Ro2XRp-Uo7IA-nEVyRzDSujO9yukG6RGsT-ks3hF01HKtq2EL8NL85KwTzJ0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. [3H]hypoxanthine incorporation after 4 hours and 24 hours of incubation of P falciparum cultures sustained with RBCs in different hydration states. The experimental protocol was similar to that indicated in Figure 4 except that [3H]hypoxanthine incorporation was measured at 4 hours (A), representing invasion efficiency, and 24 hours (B), reflecting invasion and parasite growth. Note the orders of magnitude difference in the ordinate scales between panels A and B. Panel C reports the [3H]hypoxanthine incorporation ratio (24 hours-4 hours)/4 hours, estimated from randomly paired samples. This ratio is assumed to reflect mainly parasite growth rate because the extent of [3H]hypoxanthine incorporation attributed to invasion at 4 hours is subtracted in the calculations. The right columns in each panel report [3H]hypoxanthine incorporation in untreated controls. Columns and error bars represent means and SDs of triplicate measurements.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/12/10.1182_blood-2004-12-4948/5/m_zh80120580060005.jpeg?Expires=1767701832&Signature=pV13OFa20PTDQDUi~CGR5HT0ofHN4uhql-wD-ah9Zs56GYKht3VdxKX51GkBHsk5DNGJ6CajJrkJtk5ULyUA2fWoznNF2fg1sVFWqkIJGTUMxNvqEAyVzVS-w0ZCU1fXC82VBRkW4gkkFkNU40HlbsCQbi5KKzg1wgACz7gYvg~btjrzcjgfEV9~HRocYgu~pOx-uxAPVBCTNS4RmOBvBxLph7gq1ZL16NZqxHE9tdhIHIGImNnq7B6IjzwQOUG650a4lMsi3gHeEQ-VSkT4f2vO5oHL8zx45EKoePtq4f0103CoDPvLmamsd0bh1o-3mdJJYYx-iYKcMT~tL16WXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal