The achievement of partial or complete cytogenetic remission (CCR) in response to imatinib mesylate in chronic myeloid leukemia (CML) is an indicator of favorable response to the drug.1 The current assays for prediction of response rely on immunoblot analyses of Bcr-Abl or its substrates Crkl2 or Stat5.3 However, these require large numbers of primary cells and are technically demanding. We previously showed that the inhibition of total phosphotyrosine was a reliable indicator of the effect of imatinib mesylate on BCR-ABL+ cell lines.4 We have now further developed this flow cytometric test to assess the response of primary cells to in vitro imatinib treatment.
We analyzed cryopreserved mononuclear cells from 23 patients with CML in chronic phase, 16 of whom had responded to imatinib mesylate, reaching at least a partial cytogenetic response within the first year of therapy. There was no significant difference in the interval between diagnosis and the initiation of imatinib mesylate treatment between the 2 groups. After thawing, CD34+ cells were isolated by immunomagnetic separation, kept overnight in culture, and subsequently exposed for 2 hours to doses of imatinib mesylate ranging from 0.1 to 50 μM, and intracellularly stained as described in Figure 1. The overall tyrosine phosphorylation was determined by flow cytometry and expressed as a percentage of the level of phosphorylation of the nonexposed control cells.
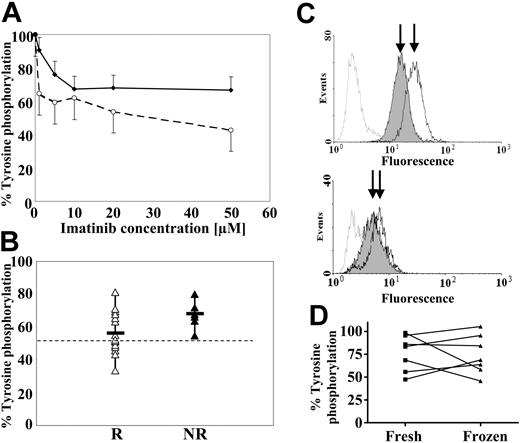
Inhibition of tyrosine phosphorylation in primary cells by imatinib mesylate. (A) Median levels of tyrosine phosphorylation in cytogenetic responders (□) and nonresponders (▪). 104 to 105 CD34+ cells per data point were treated for 2 hours with a range of imatinib mesylate concentrations, fixed in 1% paraformaldehyde, permeabilized with 0.3% saponin, and incubated with an antiphosphotyrosine (PY99; Santa Cruz, Holly Ditch Farm, United Kingdom) and a secondary fluorescent antibody (goat F(ab′)2 antimouse; Caltag, Silverstone, United Kingdom). The dose of imatinib mesylate which best distinguishes the 2 groups was found to be 20 μM. (B) Median percentage tyrosine phosphorylation of imatinib mesylate-treated cells from cytogenetic responders (R) and nonresponders (NR) compared with cells not exposed to the drug. The data show a significant difference between the median tyrosine phosphorylation of the 2 groups (Mann-Whitney test, P = .034). In about half of the patients responding to imatinib mesylate the tyrosine phosphorylation levels upon in vitro exposure to the inhibitor fell below 55% (dotted line) of that in the nonexposed cells, whereas this level of reduction was not achieved in any of the nonresponders. (C) Illustrative flow cytometric profile of 1 responder (top) and 1 nonresponder (bottom). Cells stained with the isotype control antibody (gray line) are used to adjust the flow cytometer settings, whereas cells not exposed to imatinib mesylate serve as a phosphotyrosine staining baseline for all measurements (black line). Imatinib mesylate-treated cells (shaded curve) from patients who eventually responded to imatinib mesylate show a clearly visible shift to the left, while the shift is significantly smaller for the nonresponders (as shown by the distance between the arrows pointing to the peak fluorescence of the exposed and nonexposed cells). (D) Comparison of CD34 cells used fresh or after 1 round of freezing for the assessment of total phosphotyrosine phosphorylation. From 7 extra patients, CD34+ cells from freshly collected blood were divided into 2 aliquots: 1 was placed in culture for the assay on “fresh” cells, and the second was cryopreserved and thawed later on for the same test. Each line depicts 1 paired sample of cells.
Inhibition of tyrosine phosphorylation in primary cells by imatinib mesylate. (A) Median levels of tyrosine phosphorylation in cytogenetic responders (□) and nonresponders (▪). 104 to 105 CD34+ cells per data point were treated for 2 hours with a range of imatinib mesylate concentrations, fixed in 1% paraformaldehyde, permeabilized with 0.3% saponin, and incubated with an antiphosphotyrosine (PY99; Santa Cruz, Holly Ditch Farm, United Kingdom) and a secondary fluorescent antibody (goat F(ab′)2 antimouse; Caltag, Silverstone, United Kingdom). The dose of imatinib mesylate which best distinguishes the 2 groups was found to be 20 μM. (B) Median percentage tyrosine phosphorylation of imatinib mesylate-treated cells from cytogenetic responders (R) and nonresponders (NR) compared with cells not exposed to the drug. The data show a significant difference between the median tyrosine phosphorylation of the 2 groups (Mann-Whitney test, P = .034). In about half of the patients responding to imatinib mesylate the tyrosine phosphorylation levels upon in vitro exposure to the inhibitor fell below 55% (dotted line) of that in the nonexposed cells, whereas this level of reduction was not achieved in any of the nonresponders. (C) Illustrative flow cytometric profile of 1 responder (top) and 1 nonresponder (bottom). Cells stained with the isotype control antibody (gray line) are used to adjust the flow cytometer settings, whereas cells not exposed to imatinib mesylate serve as a phosphotyrosine staining baseline for all measurements (black line). Imatinib mesylate-treated cells (shaded curve) from patients who eventually responded to imatinib mesylate show a clearly visible shift to the left, while the shift is significantly smaller for the nonresponders (as shown by the distance between the arrows pointing to the peak fluorescence of the exposed and nonexposed cells). (D) Comparison of CD34 cells used fresh or after 1 round of freezing for the assessment of total phosphotyrosine phosphorylation. From 7 extra patients, CD34+ cells from freshly collected blood were divided into 2 aliquots: 1 was placed in culture for the assay on “fresh” cells, and the second was cryopreserved and thawed later on for the same test. Each line depicts 1 paired sample of cells.
The results showed a significant difference between patients who achieved a complete or partial cytogenetic remission and those who did not (Figure 1). For the most discriminatory concentration of 20 μM imatinib mesylate, the median level of tyrosine phosphorylation inhibition in cells from the responders was 46%, as opposed to only 32% in the nonresponders (P = .034, Mann-Whitney test). None of the nonresponders had more than 45% inhibition of their total cellular phosphotyrosine content, whereas this was achieved in half of the responding patients. The molecular response to imatinib mesylate also correlated with its capacity to inhibit tyrosine phosphorylation in vitro: in none of the patients who failed to exhibit a greater than 2-log reduction in the number of BCR-ABL transcripts or a BCR-ABL/ABL ratio less than 2% was imatinib able to reduce the cellular phosphotyrosine content below 55% of the pretreatment levels, whereas 75% (6/8) of those with at least 45% inhibition of phosphotyrosine levels achieved those degrees of molecular remission. No association was found between the Sokal and Hasford scores5 and the level of decrease in tyrosine phosphorylation.
The test is able to generate reproducible data from as little as 5 × 104 CD34+ cells, can be completed within 1 day from the receipt of the sample in the laboratory, and yields similar results on fresh or cryopreserved cells (Figure 1D). In order to confirm the results of this pilot study, we initiated a prospective analysis on a larger patient group where additional clinical and molecular parameters will be assessed. If validated, this assay may facilitate decisions on whether to opt for imatinib mesylate treatment as opposed to allogeneic stem cell transplantation in the first place.
This work was funded by the Hammersmith Hospital Trust Research Committee and the Leukaemia Research Fund (United Kingdom).
We thank Efthymios Fidanis for technical assistance, Dr David Marin and Linda Fung for providing clinical information on the patients, Dr Giuseppe Saglio for cells from 1 patient, and Dr Elisabeth Buchdunger for donation of imatinib mesylate.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal