Abstract
Dyskeratosis congenita (DC) is an inherited bone marrow failure disorder characterized by abnormal skin pigmentation and nail dystrophy. We have recently described, in 10 members of a large 3-generation family, an autosomal-dominant form of DC (AD DC) that is due to a mutation in the gene-encoding human telomerase RNA (TERC), resulting in telomere shortening. In studying the immunologic consequences of TERC mutations, severe B lymphopenia and decreased immunoglobulin M (IgM) levels were noted. T cells were found to overexpress senescent markers, including CD57 and Fas receptor, and were moderately reduced in cell number. To determine whether these in vivo findings were related to cellular replicative defects, short-term cultures of AD DC lymphocytes were established to measure proliferation, mitoses, and apoptosis. AD DC lymphocytes displayed a markedly reduced proliferative capacity and increased basal apoptotic rate. Finally, telomere shortening was most prominent in third-generation subjects, and there appeared to be a correlation between telomere length and in vivo and in vitro immune findings. In summary, the observed lymphopenia and hypogammaglobulinemia in AD DC is likely a consequence of replicative failure and premature senescence of lymphocytes, supporting a role of telomerase activity in immune homeostasis.
Introduction
Classical dyskeratosis congenita (DC) is an inherited bone marrow failure disorder characterized by the triad of abnormal skin pigmentation, nail dystrophy, and mucosal leukoplakia. The predominant form of DC is X-linked and is due to mutations within the gene DKC1. The DKC1 gene product, dyskerin, is a nuclear protein that is associated with the H/ACA class of small nucleolar RNA particles responsible for the pseudouridination of rRNA (ribosomal RNA) and is a component of the telomerase complex. The principal components of the telomerase complex are telomerase RNA (TERC) and a specialized reverse transcriptase. We recently reported a large 3-generation family with an apparent autosomal-dominant (AD) inheritance pattern of DC.1 Importantly, a 74–base pair deletion at the 3′ end of the TERC gene was found in 10 members of this family. Many of these individuals displayed mild clinical features of this disease, and a macrocytic anemia was noted in nearly all patients.
Telomeres are evolutionarily conserved hexameric tandem repeats of DNA located at the tips of chromosomes and are necessary in maintaining chromosome integrity, function, and replication. Telomerase activity has also been implicated in the aging process. Telomeres will normally shorten with each successive cell division until they reach a particular length, at which stage growth arrest, or senescence, occurs. If cells continue to replicate past this stage, telomeres become critically short, resulting in disruption of chromosome integrity and cell death/apoptosis.2 Consistent with this, blocking telomerase activity by ectopic expression of a dominant-negative human telomerase reverse transcriptase (TERT) resulted in a decreased lifespan and multiple cytogenetic abnormalities in transduced T cells.3 Telomere shortening has also been well documented in lymphocytes from patients with both the X-linked and AD forms of DC, and aberrant telomere shortening in different somatic cell lineages is believed to be the underlying basis for the observed phenotype.1,4
Details on the immune system in AD DC are largely unknown. In X-linked DC, various B- and T-cell abnormalities have been reported.5 In a recent review of patients with DC, elevated, normal, and reduced immunoglobulin G (IgG) levels were noted in 38%, 45%, and 17% of patients, respectively.6 Similarly, B-cell counts were normal in 41% of patients but were reduced in 59% of patients. Recently, mutations in the DKC1 gene have been found in Hoyeraal-Hreidarsson syndrome, suggesting this may be a particularly severe form of X-linked DC. This multisystem disorder is characterized by cerebellar hypoplasia, aplastic anemia, and a severe immune deficiency, including B lymphopenia and low immunoglobulin levels.7 Likewise, hypomorphic Dkc1-mutant mice displayed significant lymphopenia by 6 months of age.8 Similarly, a reduction in the B-lymphoid compartment and low antigen-specific IgM levels were recently described in mTerc-/- mice.9
The current study is the first complete description of immune characteristics in AD DC. Consistent with murine models, AD DC subjects also displayed a similar pattern of immune abnormalities, including lymphopenia and hypogammaglobulinemia. To understand the mechanisms underlying the observed clinical and laboratory findings of lymphopenia in AD DC, a series of in vitro experiments was carried out. AD DC lymphocytes were functional, though they displayed a senescent phenotype. Additionally, T lymphocytes had a markedly reduced proliferative capacity, altered mitotic profile, and predisposition to apoptosis in long-term culture. These data support a critical role of TERC in lymphocyte survival and homeostasis.
Patients, materials, and methods
Patients
The study was conducted at the University of Iowa from October 2002 to August 2003 on multiple family members with AD DC, as recently detailed.1 The University of Iowa Institutional Review Board approved this study and all subjects gave informed consent. Molecular studies on peripheral blood confirmed the same deletion mutation of TERC for 10 family members unique patient number (UPN) 2-11. Two of the patients, UPNs 1 and 12, died prior to molecular and immune testing, though they displayed typical clinical and laboratory features of DC. All study patients were free of clinically apparent infection at the time of immune analyses.
Lymphocyte separation and immunophenotyping
Peripheral blood mononuclear cells (PBMCs) from AD DC subjects and healthy volunteers were isolated by Ficoll-Hypaque gradient centrifugation. Following isolation, cells were plated at a density of 1 × 106/mL to 2 × 106/mL and incubated in tissue culture flasks at 37°C in 5% CO2 for 2 hours to remove adherent cells. The immunophenotyping experiments were performed as described previously.10
T-cell activation and blastogenic assays
To assess T-cell function by fluorescence-activated cell sorter (FACS), peripheral blood leukocyte (PBLs) were placed in Costar (Corning Incorporated, Corning, NY) plates coated with biotinylated anti-CD3 (PharMingen, San Diego, CA). Following an 18-hour incubation, cells were washed and then stained with a combination of antibodies, including anti-CD4–fluorescein isothiocyanate (FITC)/anti-CD69–phycoerythrin (PE), and anti-CD8–FITC/anti-CD69–PE. Fluorescence intensity was calculated and compared with control values, as previously described.11 In addition, PBLs were cultured in triplicate in microtiter wells at 4 × 105 cells per well, with medium alone or with the indicated stimuli. Cells were exposed either to mitogens (concanavalin A [Con A], phytohemagglutinin [PHA], pokeweed mitogen [PWM], immobilized anti-CD3) for 3 days or to specific antigens (tetanus, mumps, alloantigen) for 6 days. On the last day of stimulation, wells were pulsed with [3H] thymidine, and after 4 hours, [3H] incorporation was assessed by β scintography.12
Cell culture conditions
For long-term cultures, Ficoll-separated lymphocytes were maintained in complete RPMI media (10% fetal calf serum, penicillin 1000 U/mL, streptomycin 1000 U/mL, and glutamine 20 mM) supplemented with soluble anti-CD3 (1 μg/mL), anti-CD28 (0.5 μg/mL), and interleukin 2 (IL-2; 50 U/mL). Media were changed and cultures were split every 3 to 4 days to maintain a cell density ranging from 1 × 106/mL to 2 × 106/mL.
Proliferation assays
Total lymphocyte counts were recorded every 1 to 2 days, as determined by a hemocytometer under low-power (× 100) microscopy (Nikon Abbe 1.25, Nikon Corporation, Tokyo, Japan). Only live cells were counted, as discriminated by trypan blue dye exclusion. The proliferative index is expressed as percent fold difference in total cell number, over time, relative to healthy age-matched control cells cultured under identical conditions.
Mitotic profile
Freshly isolated lymphocytes from patients and age-matched controls were loaded with CFSE (5-6-carboxyfluorescein diacetate succinimidyl ester; 2.5 μM), then rinsed as per manufacturer's instructions (Molecular Probes, Eugene, OR). At regular intervals, cells were removed from culture, resuspended in 1 × phosphate-buffered saline (PBS), then analyzed by flow cytometry using the FL1 channel (530/30 band path) as previously described.10 Using standard gating, the percentage of lymphocytes undergoing specific numbers of replications (mitoses) was calculated at day 4 and day 8 in culture. Data on patients are expressed as a percentage of cells, relative to controls, undergoing varying numbers (0, 1-2, or 3 or more) of mitoses at the designated day in culture.
Apoptosis assays
Lymphocytes from patients and age-matched controls were removed from culture between days 10 and 14, placed in fresh 24-well Costar plates, then incubated for 18 hours with soluble anti-Fas antibody (1 μg/mL), kindly provided by Dr Oskar Rokhlin (University of Iowa). Basal apoptosis was measured in similarly cultured cells that were not exposed to anti-Fas. Cells were washed and stained with annexin V–FITC/propidium iodide (PI) according to the manufacturer's protocol (ApoAlert Annexin V–FITC Apoptosis Kit; Clontech, Palo Alto, CA). Flow cytometry was performed with a FACScan, and Cellquest V3.2 software (Becton Dickinson, San Jose, CA) was used for acquisition and analysis of data. Forward scatter (FSC) and orthogonal scatter (SSC) were collected using linear amplification. Annexin-FITC and PI fluorescence were collected using log amplification and 10 000 events were collected. A gate was placed around the cell population on the basis of FSC and SSC to eliminate small debris and aggregates. The gate was large enough so that apoptotic cells were not eliminated. Cells satisfying the gate were analyzed using quadrant statistics in an annexin V–FITC versus PI dual-parameter histogram. Data for basal and Fas-mediated apoptosis expressed as percent difference relative to age-matched controls.
Telomere assays
Telomere length of cells was measured by flow cytometry using the FITC-conjugated peptide nucleic acid (PNA) probe. Relative telomere length (RTL) was determined by comparing isolated test cells with a control cell line, GM03671C (Coriell Institute of Medical Research, Camden, NJ). This tetraploid cell line served as an internal control, with telomere length of subjects normalized to the telomere length of control cells. PBMCs from AD DC subjects and the control cells (cell line GM 03671C) were washed in PBS and mixed 1:1. The mixture and was resuspended in 300 μL of hybridization solution containing 70% formamide with either no probe (unstained control) or with a fluorescein-conjugated telomere PNA probe. The cells were heated for 10 minutes at 82°C for DNA denaturation. Hybridization was performed overnight at room temperature in the dark. Cells were washed to remove unbound probe and resuspended in staining solution containing PI and RNAse for DNA staining and incubated at 2°C to 8°C for 2 to 3 hours in the dark. Samples were then analyzed on a FACScan using logarithmic scale FL1-H for probe fluorescence and linear scale FL3-H for DNA staining. Samples that hybridized with the telomere PNA probe/FITC exhibited a fluorescence signal in FL1, which was higher than the background/auto-fluorescence signal obtained from the sample of the same cells hybridized with the hybridization solution without probe. Fluorescence intensity of these cells was then used to calculate the RTL of the sample cells compared with the control cells.
Statistics
Data analyses primarily involved descriptive statistics and graph summaries. The 1-sample t test was used to determine whether the mean relative fold increase for subjects compared with age-matched controls was significantly different from 1.
Results
Patient population and clinical/hematologic findings
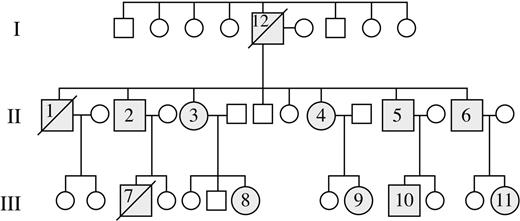
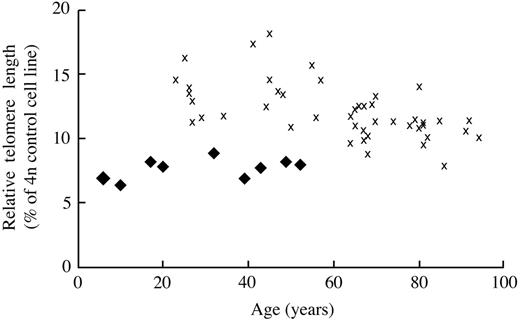
Figure 1 represents the pedigree of our study population. Molecular analyses were performed on 10 patients, UPN 2 to UPN 11, and these individuals were found to have a 74-bp deletion of the 3′ end of mature TERC RNA.1 UPN 12 was the likely proband (generation 1) and had a classic phenotype of DC, including markedly dystrophic nails, hypopigmented skin, premature graying of hair, cirrhosis, and pulmonary fibrosis. He had a history of mild thrombocytopenia and megaloblastic anemia for 10 years prior to his death at 68 years of age from sepsis and progressive pulmonary fibrosis. UPN 1 (generation 2) also had similar clinical characteristics as his father, including dystrophic nails and hypopigmentation. Additionally he had a long-standing history of severe thrombocytopenia and megaloblastic anemia of 25 years' duration. He died at 47 years of age from complications related to cirrhosis and pulmonary fibrosis. UPN 7 (generation 3) was the index case and was given the diagnosis of idiopathic aplastic anemia at 12 years of age. He was initially treated with immunosuppressive therapy, including antithymocyte globulin and cyclosporin, without improvement in blood counts. His blood counts eventually stabilized on danazol therapy, which was continued for 5 years. A clinical diagnosis of DC was made at 18 years of age based upon abnormal skin pigmentation, dystrophic nails, and pancytopenia. Due to platelet transfusion dependence, along with the development of myelodysplastic syndrome and the cytogenetic finding of monosomy 7 in the bone marrow, the patient was referred for allogeneic stem cell transplantation. At 21 years of age, he underwent a fully myeloablative matched unrelated allogeneic bone marrow transplantation. He died 25 days following transplantation from multiorgan failure, sepsis, and pulmonary hemorrhage. Prior to his death, blood samples were collected from him and family members, confirming the mutation of the TERC gene. The remaining patients from the second and third generation have been followed on a semiannual basis for the past 2 years, and the clinical features and hematologic findings are outlined in Table 1. Of note, skin and nail findings have appeared earlier in patients in the third generation relative to those reported by second-generation members. Similarly, no second-generation patients had laboratory features or clinical symptoms suggestive of cytopenia develop prior to 20 years of age, while 3 of 5 third-generation members had documented thrombocytopenia by 8 to 13 years of age. Finally, the disease is not static, and there appears to be progressive cytopenia and telomere shortening in many third-generation individuals over the past 2 years (data not shown). While the initial description of shortened telomeres in this family used Southern blotting technique,1 our current study used a highly sensitive TeloFISH assay that measures average telomere length within the lymphocyte population (Figure 2). As noted, telomere shortening appears to be more pronounced in third-generation family members relative to those in the second generation.
Pedigree of AD DC family with TERC mutation. Molecular analyses were performed on 10 patients, UPN 2 to UPN 11, and all shared the same 74–base pair deletion of the 3′ end of the mature TERC RNA. UPNs 2 and 12 had classical features of this disease and died of hematologic and pulmonary complications prior to the study onset.
Pedigree of AD DC family with TERC mutation. Molecular analyses were performed on 10 patients, UPN 2 to UPN 11, and all shared the same 74–base pair deletion of the 3′ end of the mature TERC RNA. UPNs 2 and 12 had classical features of this disease and died of hematologic and pulmonary complications prior to the study onset.
Clinical and hematologic features of AD DC
Generation . | UPN . | Age, y . | Skin/nail findings . | WBC/(mm3) . | ANC/(mm3) . | Hemoglobin level, g/dL . | MCV, fL . | Platelet count, × 103/μL . |
|---|---|---|---|---|---|---|---|---|
| I | 12 | 68* | +/+ | 8000 | NA | 14.7 | 100† | 69‡ |
| II | 1 | 45* | +/+ | 2200 | 1056‡ | 10.6‡ | 117† | 16‡ |
| II | 2 | 50 | +/+ | 3910 | 1900 | 13.0 | 91 | 293 |
| II | 3 | 46 | +/+ | 3900 | 2391 | 12.3‡ | 104† | 140‡ |
| II | 4 | 41 | +/+ | 5100 | 2820 | 12.7‡ | 103† | 196 |
| II | 5 | 37 | +/+ | 5300 | 2760 | 14.7 | 95† | 210 |
| II | 6 | 30 | +/+ | 5000 | 3220 | 12.5‡ | 104† | 94‡ |
| III | 7 | 22* | +/+ | 4400 | 2068 | 11.4‡ | 118† | 38‡ |
| III | 8 | 18 | +/+ | 3220 | 1420 | 14.6 | 99† | 83‡ |
| III | 9 | 15 | –/+ | 5300 | 2690 | 13.0 | 90 | 255 |
| III | 10 | 8 | –/– | 5400 | 2376 | 12.1‡ | 89 | 147‡ |
| III | 11 | 4 | –/– | 9900 | 5500 | 12.5 | 81 | 228 |
Generation . | UPN . | Age, y . | Skin/nail findings . | WBC/(mm3) . | ANC/(mm3) . | Hemoglobin level, g/dL . | MCV, fL . | Platelet count, × 103/μL . |
|---|---|---|---|---|---|---|---|---|
| I | 12 | 68* | +/+ | 8000 | NA | 14.7 | 100† | 69‡ |
| II | 1 | 45* | +/+ | 2200 | 1056‡ | 10.6‡ | 117† | 16‡ |
| II | 2 | 50 | +/+ | 3910 | 1900 | 13.0 | 91 | 293 |
| II | 3 | 46 | +/+ | 3900 | 2391 | 12.3‡ | 104† | 140‡ |
| II | 4 | 41 | +/+ | 5100 | 2820 | 12.7‡ | 103† | 196 |
| II | 5 | 37 | +/+ | 5300 | 2760 | 14.7 | 95† | 210 |
| II | 6 | 30 | +/+ | 5000 | 3220 | 12.5‡ | 104† | 94‡ |
| III | 7 | 22* | +/+ | 4400 | 2068 | 11.4‡ | 118† | 38‡ |
| III | 8 | 18 | +/+ | 3220 | 1420 | 14.6 | 99† | 83‡ |
| III | 9 | 15 | –/+ | 5300 | 2690 | 13.0 | 90 | 255 |
| III | 10 | 8 | –/– | 5400 | 2376 | 12.1‡ | 89 | 147‡ |
| III | 11 | 4 | –/– | 9900 | 5500 | 12.5 | 81 | 228 |
Complete blood counts and physical examinations were performed on all subjects except UPN 12 and UPN 1, where information was extracted from medical records. Skin findings included hypopigmentation or hyperpigmentation, while nail findings included ridging and dystrophic changes. WBC indicates white blood cell count; ANC, absolute neutrophil count; and MCV, mean corpuscular volume.
Dead
Value greater than 2 standard deviations above age-matched population
Value greater than 2 standard deviations below age-matched population
TeloFlow-FISH (fluorescence in situ hybridization) was performed to measure the relative telomere length, as described in “Patients, materials, and methods.” The relative telomere length in lymphocytes isolated from AD DC subjects ( ) or healthy controls (✖) is presented on the y-axis, with age in years on the x-axis.
) or healthy controls (✖) is presented on the y-axis, with age in years on the x-axis.
TeloFlow-FISH (fluorescence in situ hybridization) was performed to measure the relative telomere length, as described in “Patients, materials, and methods.” The relative telomere length in lymphocytes isolated from AD DC subjects ( ) or healthy controls (✖) is presented on the y-axis, with age in years on the x-axis.
) or healthy controls (✖) is presented on the y-axis, with age in years on the x-axis.
Humoral and cellular immune abnormalities
Prior studies in X-linked DC and AD DC demonstrated telomere shortening in lymphocytes.1,4 Nonetheless, the precise role of telomere length and telomerase activity in immune function and lymphocyte survival has yet to be elucidated. Studies were thus undertaken to assess the qualitative and quantitative effect of the TERC mutation on both the humoral and cellular immune systems of AD DC subjects. As shown in Table 2, immunoglobulin levels were consistently abnormal with low IgM and elevated IgA serum concentrations. The only exception to this was UPN 11, the youngest subject. In 4 of 10 individuals, IgG was also elevated. To determine if the diminished IgM levels were a consequence of decreased numbers of B cells, immunophenotyping of peripheral blood was undertaken. CD19+ B-cell counts were markedly decreased in nearly all patients, with the only exceptions being noted in 2 of the youngest patients (UPNs 9 and 11). Further immunophenotyping experiments revealed an increased percentage of CD3+ T cells, although the absolute T-cell counts (data not shown) were in the normal to low range due to the relatively low absolute lymphocyte counts. Similarly, the percentage of natural killer (NK) cells in AD DC subjects was in the low normal range. T-cell subsets showed no significant abnormalities in the percentage of CD4 and CD8 cells, and the CD4/CD8 ratio ranged from 1:1 to 3:1 (exception was UPN 7). However, there was increased expression of T-cell surface activation/senescent markers in many patients, including CD4/CD95 and CD8/CD57. As noted in Table 3, T-lymphocyte function was normal, as measured by anti-CD3–induced up-regulation of cell surface activation marker CD69. T- and B-cell mitogenic responses (PHA, Con A, and PWM) were also intact, as measured in a standard blastogenic assay. Finally, blastogenic assays were used to quantitate antigen-specific T-cell reactivity (tetanus, mumps, and alloantigen), and responses were comparable with those of control values.
Immunophenotype and immunoglobulin levels in AD DC
UPN . | Age, y . | ALC, (mm3) . | CD19, (mm3) . | CD3, % of total lymphocytes . | CD4, % of total lymphocytes . | CD8, % of total lymphocytes . | NK, % of total lymphocytes . | CD4/95, % of total lymphocytes . | CD8/57, % of total lymphocytes . | IgA, mg/dL . | IgM, mg/dL . | IgG, mg/dL . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45* | 770† | 77† | 87 | NA | NA | 2 | NA | NA | NA | NA | NA |
| 2 | 50 | 1259 | 88† | 76 | 65 | 18 | 7 | 50 | 39 | 782‡ | 44† | 2444‡ |
| 3 | 46 | 1172 | 70† | 78 | 66 | 13 | 14 | 44 | 41 | 489‡ | 26† | 1580 |
| 4 | 41 | 1810 | 90† | 84 | 59 | 25 | 7 | 39 | 25 | 492‡ | 34† | 1223 |
| 5 | 37 | 1720 | 86† | 87 | 44 | 41 | 5 | 52 | 42 | 462‡ | 48† | 2033‡ |
| 6 | 30 | 1350 | 95† | 83 | 61 | 20 | 7 | 43 | 33 | 319 | 27† | 1806‡ |
| 7 | 22* | 2254 | 23† | 96 | 15 | 79 | 1 | NA | NA | 489‡ | 59† | 915 |
| 8 | 18 | 1392 | 84† | 74 | 42 | 26 | 11 | 53 | 50 | 353 | 61 | 1224 |
| 9 | 15 | 2020 | 242 | 68 | 35 | 25 | 7 | 30 | 14 | 503‡ | 38† | 1112 |
| 10 | 8 | 1836 | 128 | 70 | 42 | 23 | 11 | 59 | 29 | 537‡ | 49 | 1507‡ |
| 11 | 4 | 3560 | 854 | 50 | 34 | 18 | 8 | 36 | 9 | 123 | 113 | 910 |
| Control | 4-10 | 1200-3600 | 168-828 | 60-76 | 31-47 | 18-35 | 4-17 | 25-53 | 10-30 | 32-241 | 37-185 | 491-1375 |
| Control | > 15 | 875-3300 | 122-690 | 49-80 | 23-48 | 13-41 | 6-37 | 18-33 | 10-30 | 68-378 | 60-263 | 694-1618 |
UPN . | Age, y . | ALC, (mm3) . | CD19, (mm3) . | CD3, % of total lymphocytes . | CD4, % of total lymphocytes . | CD8, % of total lymphocytes . | NK, % of total lymphocytes . | CD4/95, % of total lymphocytes . | CD8/57, % of total lymphocytes . | IgA, mg/dL . | IgM, mg/dL . | IgG, mg/dL . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45* | 770† | 77† | 87 | NA | NA | 2 | NA | NA | NA | NA | NA |
| 2 | 50 | 1259 | 88† | 76 | 65 | 18 | 7 | 50 | 39 | 782‡ | 44† | 2444‡ |
| 3 | 46 | 1172 | 70† | 78 | 66 | 13 | 14 | 44 | 41 | 489‡ | 26† | 1580 |
| 4 | 41 | 1810 | 90† | 84 | 59 | 25 | 7 | 39 | 25 | 492‡ | 34† | 1223 |
| 5 | 37 | 1720 | 86† | 87 | 44 | 41 | 5 | 52 | 42 | 462‡ | 48† | 2033‡ |
| 6 | 30 | 1350 | 95† | 83 | 61 | 20 | 7 | 43 | 33 | 319 | 27† | 1806‡ |
| 7 | 22* | 2254 | 23† | 96 | 15 | 79 | 1 | NA | NA | 489‡ | 59† | 915 |
| 8 | 18 | 1392 | 84† | 74 | 42 | 26 | 11 | 53 | 50 | 353 | 61 | 1224 |
| 9 | 15 | 2020 | 242 | 68 | 35 | 25 | 7 | 30 | 14 | 503‡ | 38† | 1112 |
| 10 | 8 | 1836 | 128 | 70 | 42 | 23 | 11 | 59 | 29 | 537‡ | 49 | 1507‡ |
| 11 | 4 | 3560 | 854 | 50 | 34 | 18 | 8 | 36 | 9 | 123 | 113 | 910 |
| Control | 4-10 | 1200-3600 | 168-828 | 60-76 | 31-47 | 18-35 | 4-17 | 25-53 | 10-30 | 32-241 | 37-185 | 491-1375 |
| Control | > 15 | 875-3300 | 122-690 | 49-80 | 23-48 | 13-41 | 6-37 | 18-33 | 10-30 | 68-378 | 60-263 | 694-1618 |
Data are representative of 3 separate analyses. All subjects were free of active infection at the time of analysis. ALC indicates absolute lymphocyte count; NA indicates not available.
Dead
Value greater than 2 standard deviations below age-matched population
Value greater than 2 standard deviations above age-matched population
Cellular immune function in AD DC
. | Anti-CD3* . | . | Antigen response, cpm† . | . | . | Mitogen response, cpm† . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | CD4/69 . | CD8/69 . | Tetanus . | Mumps . | Alloantigen . | PHA . | ConA . | Anti-CD3 . | PWM . | ||||||
| 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||
| 2 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||
| 3 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||
| 4 | WNL | WNL | 385 | 205 | 12958 | 66894 | 57480 | 26873 | 40911 | ||||||
| 5 | WNL | WNL | 1098 | 301 | 26206 | 88970 | 77125 | 25525 | 42825 | ||||||
| 6 | WNL | WNL | 1646 | 961 | 57997 | 114714 | 92272 | 46346 | 66195 | ||||||
| 7 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||
| 8 | WNL | WNL | NA | NA | NA | NA | NA | NA | NA | ||||||
| 9 | WNL | WNL | 1642 | 215 | 16697 | 85719 | 84726 | 28304 | 43580 | ||||||
| 10 | WNL | WNL | 7897 | 2976 | 9222 | 110164 | 107465 | 46529 | 39864 | ||||||
| 11 | WNL | WNL | 6721 | 1390 | 11632 | 65690 | 43971 | 30643 | 43010 | ||||||
| Control | WNL | WNL | 2048 | 533 | 9707 | 52131 | 50201 | 13779 | 34709 | ||||||
. | Anti-CD3* . | . | Antigen response, cpm† . | . | . | Mitogen response, cpm† . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | CD4/69 . | CD8/69 . | Tetanus . | Mumps . | Alloantigen . | PHA . | ConA . | Anti-CD3 . | PWM . | ||||||
| 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||
| 2 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||
| 3 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||
| 4 | WNL | WNL | 385 | 205 | 12958 | 66894 | 57480 | 26873 | 40911 | ||||||
| 5 | WNL | WNL | 1098 | 301 | 26206 | 88970 | 77125 | 25525 | 42825 | ||||||
| 6 | WNL | WNL | 1646 | 961 | 57997 | 114714 | 92272 | 46346 | 66195 | ||||||
| 7 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||
| 8 | WNL | WNL | NA | NA | NA | NA | NA | NA | NA | ||||||
| 9 | WNL | WNL | 1642 | 215 | 16697 | 85719 | 84726 | 28304 | 43580 | ||||||
| 10 | WNL | WNL | 7897 | 2976 | 9222 | 110164 | 107465 | 46529 | 39864 | ||||||
| 11 | WNL | WNL | 6721 | 1390 | 11632 | 65690 | 43971 | 30643 | 43010 | ||||||
| Control | WNL | WNL | 2048 | 533 | 9707 | 52131 | 50201 | 13779 | 34709 | ||||||
cpm indicates counts per minute; NA, not available; and WNL, within normal limits.
FACS was used to assay T-cell subset function, measuring anti-CD3-induced up-regulation of cell surface activation marker CD69
Antigen and mitogen responses were determined by blastogenic assays
Cellular proliferation and mitotic profile
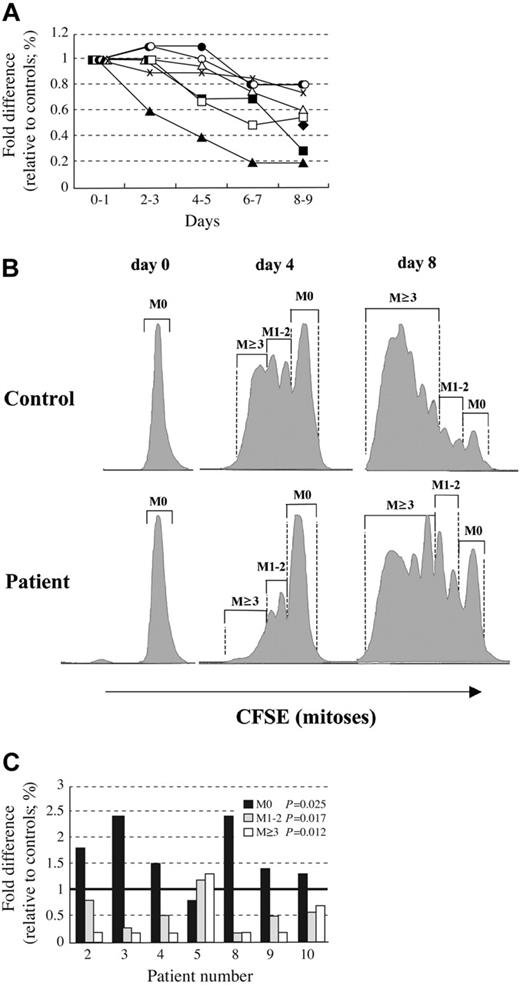
Lymphocytes, particularly B cells, are known to have a high proliferative capacity and undergo clonal expansion in response to antigenic exposure. Human T and B cells undergo telomere shortening with age, and telomerase activity can be up-regulated at defined stages of differentiation and after activation.13 Given the previous findings of telomere length shortening in DC lymphocytes, in vitro studies were undertaken to determine the replicative capacity of AD DC lymphocytes. Over the course of an 8-week period, mononuclear cells from 7 DC subjects and age-matched healthy controls were collected, separated, and set up in long-term culture. Initial experiments attempted to selectively expand B cells, but due to the marked B lymphopenia in these subjects, sufficient cell numbers could not be obtained. Thus, subsequent experiments used culture conditions promoting T-cell growth. Immunophenotyping assays were performed on the first day to ensure that equal numbers of T cells were in both control and AD DC cultures. While there was some variability in percentage of B cells on the first day of culture, after 4 days in culture more than 95% of cells were CD3+. Cells were counted every 1 to 2 days, and, as noted in Figure 3A, cell growth was not significantly different in control versus AD DC lymphocytes after 2 to 3 days in culture. However, by 6 days in culture, a significant (P = .008) reduction in cell expansion was noted for AD DC lymphocytes. This trend continued until cultures were terminated at day 10. In addition to measuring proliferative capacity, simultaneous experiments were also set up at the onset to assess the cellular mitotic profile. Lymphocytes were loaded with CFSE, an intracellular protein stain, and the number of cells divisions, or mitoses, were determined by following fluorescent staining intensity in the FL1 wavelength. As noted in Figure 3B, the majority of control cells had undergone at least 1 mitotic division by day 4 and many had undergone 3 divisions. In contrast, only a small percentage of DC lymphocytes had undergone multiple cell divisions. Figure 3C summarizes the mitotic profiles for cells cultured 4 days, and a similar pattern was also observed in 8-day cultures (data not shown).
Diminished in vitro proliferation and altered mitotic profiles of AD DC lymphocytes. Lymphocytes collected from various AD DC subjects (UPN) and age-matched controls were placed in long-term culture supplemented with soluble anti-CD3 (1 μ g/mL), anti-CD28 (0.5 μ g/mL), and IL-2 (50 U/mL). For mitoses determinations, cells were loaded with CFSE as per manufacturers instructions. (A) Proliferation was quantitated by counting live cells and is expressed as percent fold difference in total cell number, over time, relative to healthy age-matched controls (unique patients represented by different symbols). P values (as calculated by comparing mean value relative to control to determine if significantly different than 1 at each time point) were significant at days 6 to 7 (P = .008) and days 8 to 9 (P = .0006). (B) Example of gating strategy in FL1 wavelength for determining number of mitoses (M = 0, 1-2, or ≥ 3). (C) Mitotic profile for 7 AD DC subjects, expressed as a percentage of gated cells within the entire cell population undergoing varying numbers (0, 1-2, or ≥ 3) of mitoses on day 4 in culture, relative to control values. The P values (as calculated from the average percent of cells from the patients versus the controls in each mitotic grouping to determine if mean value relative to control was significantly different from 1) are displayed in the inserted box. Bold line on y axis represents control value.
Diminished in vitro proliferation and altered mitotic profiles of AD DC lymphocytes. Lymphocytes collected from various AD DC subjects (UPN) and age-matched controls were placed in long-term culture supplemented with soluble anti-CD3 (1 μ g/mL), anti-CD28 (0.5 μ g/mL), and IL-2 (50 U/mL). For mitoses determinations, cells were loaded with CFSE as per manufacturers instructions. (A) Proliferation was quantitated by counting live cells and is expressed as percent fold difference in total cell number, over time, relative to healthy age-matched controls (unique patients represented by different symbols). P values (as calculated by comparing mean value relative to control to determine if significantly different than 1 at each time point) were significant at days 6 to 7 (P = .008) and days 8 to 9 (P = .0006). (B) Example of gating strategy in FL1 wavelength for determining number of mitoses (M = 0, 1-2, or ≥ 3). (C) Mitotic profile for 7 AD DC subjects, expressed as a percentage of gated cells within the entire cell population undergoing varying numbers (0, 1-2, or ≥ 3) of mitoses on day 4 in culture, relative to control values. The P values (as calculated from the average percent of cells from the patients versus the controls in each mitotic grouping to determine if mean value relative to control was significantly different from 1) are displayed in the inserted box. Bold line on y axis represents control value.
Apoptosis
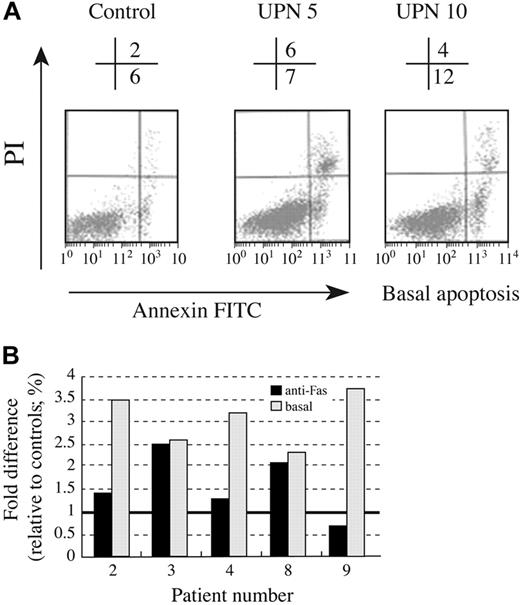
Programmed cell death, or apoptosis, controls lymphocyte homeostasis and data suggest telomerase activity may also play a role in this process.2 Given the in vivo findings of lymphopenia and the in vitro findings of reduced proliferation of AD DC lymphocytes, we hypothesized that AD DC lymphocytes might have an increased susceptibility to apoptosis or apoptotic stimuli. To address this possibility, 5 AD DC subjects and age-matched controls were studied. Lymphocytes were cultured for 10 days in T-cell growth media (similar to proliferation assays), then treated with anti-Fas for an additional 18 hours. To assess basal apoptosis, cells were left untreated (eg, no anti-Fas) for the same 18-hour period. Cells were then stained with annexin V–FITC and apoptosis was quantitated by FACS (Figure 4A). Basal and Fas-mediated apoptosis of lymphocytes from 5 AD DC subjects is presented in Figure 4B. A significant increase in basal apoptosis was noted in all study subjects (P = .002). Lymphocytes isolated from 2 of 5 subjects also displayed increased Fas-induced apoptosis. Thus, while the Fas signaling pathway appears intact, there may be an underlying predisposition to apoptosis related to the senescent stage of AD DC lymphocytes.
Increased apoptosis in AD DC lymphocytes. Lymphocytes from 5 AD DC subjects and age-matched controls were examined for apoptosis by FACS. (A) Sample FACS profile of basal apoptosis for lymphocytes cultured 10 days, representing 2 AD DC subjects and 1 control. The percentage of apoptotic cells (FITC positive) is shown above the FACS histogram. (B) Summary data for 5 AD DC subjects. Basal and Fas-mediated apoptosis is expressed as percent difference of FITC gated AD DC cells relative to age-matched controls, as described in “Patients, materials, and methods.” Bold line on y axis represents control level.
Increased apoptosis in AD DC lymphocytes. Lymphocytes from 5 AD DC subjects and age-matched controls were examined for apoptosis by FACS. (A) Sample FACS profile of basal apoptosis for lymphocytes cultured 10 days, representing 2 AD DC subjects and 1 control. The percentage of apoptotic cells (FITC positive) is shown above the FACS histogram. (B) Summary data for 5 AD DC subjects. Basal and Fas-mediated apoptosis is expressed as percent difference of FITC gated AD DC cells relative to age-matched controls, as described in “Patients, materials, and methods.” Bold line on y axis represents control level.
Discussion
Our study is the first to characterize the immune abnormalities in individuals with AD DC. A variable immune deficiency has also been described in the X-linked form of the disease.5,6 In some cases of X-linked DC, the immune deficiency is very severe and has been categorized as “T+B-NK SCID,”14 where SCID indicates severe combined immunodeficiency. Since reporting the TERC mutation as the underlying molecular basis for AD DC1 we have been closely following a large 3-generation–affected kindred. Although our original report on AD DC described these individuals as having a milder phenotype relative to the X-linked form of this disease, we have seen progressive clinical and hematologic changes in our subjects, particularly for those in the third generation. While hematologic data were not available on second-generation DC subjects during their youth, they had no symptoms of cytopenia. This contrasts with those in the third generation, where symptoms of thrombocytopenia have been noted in 3 of 6 subjects prior to 15 years of age. Additionally, 1 patient developed severe aplastic anemia and died following an allogeneic bone marrow transplantation. Of note, third-generation patients also have the shortest relative telomere lengths. This pattern of a more severe disease phenotype in later generations is consistent with that noted in mTerc-/- mice, where clinical features were not evident until the fourth generation.9,15 The observation that the clinical phenotype of AD DC is progressive in subsequent generations has recently been reported for other AD DC families as well. This phenomenon is referred to as “anticipation” and appears to correlate with telomere length.16,17 Thus, one might expect to see a disease phenotype in later-generation AD DC subjects that is more severe, appears earlier, and more closely resembles that noted in X-linked DC or even Hoyeraal-Hreidarsson syndrome.
In addition to the clinical observations and progressive cytopenia, we have also noted that nearly all AD DC subjects had marked lymphopenia and immune abnormalities. These immune abnormalities were primarily humoral, with a significant decrease in B-cell number, low IgM serum levels, and elevated IgA levels in 9 of 10 subjects tested. A variable pattern of similar immune abnormalities5,6,14 has previously been observed in X-linked DC. The findings of this study therefore further develop the functional link between these 2 subtypes of DC. It is noteworthy that despite laboratory evidence of aberrant humoral immunity, most patients have not had a long-standing history of severe or recurrent infections. Nonetheless, infections were a primary cause of death in 2 individuals, though they also had concomitant hepatic and pulmonary dysfunction. It is not entirely clear at this stage why AD DC patients have a selective IgM deficiency. Not surprisingly, our data are consistent with animal models of defective telomerase activity. In animals expressing a mutated dyskerin gene, Dkc1m, a relative lymphopenia was observed, with a more pronounced decrease in B lymphocytes (relative to T lymphocytes) in bone marrow.8 Similarly, in mTerc-/- animals, B cells were lost from lymph node germinal centers, and antigen-specific IgM titers were lower relative to antigen-specific IgG levels.9
Despite the hypogammaglobulinemia and lymphopenia, lymphocyte function appeared normal. AD DC lymphocytes responded normally to various T- and B-cell mitogens in a blastogenic assay; up-regulated surface activation marker CD69 in response to anti-CD3 stimulation; and, in addition, IL-2, IL-4, and interferongamma mRNA synthesis was intact (data not shown). These data differs slightly from that found in X-linked DC, where approximately 50% of patients had reduced mitogenic responses.6 It will be important to study other AD DC families before it can be concluded that this is a consistent feature in all AD DC patients. Lymphocyte function was also examined in mTerc-/- mice, and splenocytes from these animals had an impaired proliferative capacity to both T- and B-cell–specific mitogens.9 Nonetheless, our findings of intact B- and T-cell mitogenic responses are consistent with the clinical history of AD DC subjects, where few individuals have developed opportunistic or recurrent infections.
Telomeres and telomerase have been shown to play a central role in cellular proliferation and differentiation. In fact, telomerase activity is tightly regulated during differentiation of B cells in tonsillar germinal centers and is normally up-regulated in activated mature T cells.13,18 Experiments were thus undertaken to determine the relative role of telomere shortening and TERC gene mutations in lymphocyte replication. Not surprisingly, AD DC lymphocytes from nearly all subjects had a decreased growth velocity in vitro and underwent fewer mitoses relative to age-matched control cells. The fact that differences were more pronounced at later time points in culture is suggestive of AD DC lymphocytes having reached their nondividing state of replicative senescence. While telomere length was quantitated on freshly isolated lymphocytes from all AD DC subjects and was significantly shorter relative to controls, we do not know the telomere length of AD DC lymphocytes cultured long-term. It is known that in some somatic cells, such as fibroblasts, a decrease in in vitro proliferation is accompanied by a shortening of telomeres.19 However, this may be different in lymphocytes, and, of note, a recent study showed no direct correlation between telomere length, telomerase activity, and in vitro B-cell proliferation.20 There is strong data, however, to suggest that telomerase activity is a limiting factor for the lifespan of T cells, as memory T cells have shorter telomeres and a reduced proliferative capacity relative to naive T cells.21 Furthermore, ectopic expression of human TERT immortalizes T cells, and expression of a dominant-negative TERT construct results in decreased lifespan of T cells.3,22,23
The role of telomere length in regulating replicative senescence is currently being investigated by a number of groups.2 Normal cells are capable of a finite number of cell divisions prior to entering replicative senescence. The findings in AD DC of lymphopenia and reduced in vitro cellular proliferation may in fact suggest that these cells have reached replicative senescence. The phenotypes associated with replicatively senescent T cells are not well defined but are generally attributed to lack of CD28 or overexpression of CD57.24,25 CD57 is also up-regulated in a variety of disease states, including HIV infection, rheumatoid arthritis, and autoimmune lymphoproliferative syndrome.24,26,27 It is noteworthy that CD57 was up-regulated in freshly isolated CD8+ T cells from many AD DC subjects.
While the progression from replicative senescence to apoptosis is implied, some senescent cells may remain metabolically active for years prior to death.28 It has also been shown that telomere shortening is associated with an increase in apoptosis as well as senescence in human fibroblasts.29 We examined apoptosis in freshly isolated lymphocytes and long-term–cultured T cells. Interestingly, a subtle increase in basal apoptosis was found in freshly isolated lymphocytes from AD DC subjects (data not shown). When cells were cultured in vitro for 10 days, a significant increase in basal apoptosis was noted in all subjects tested. In addition, Fas-induced apoptosis was increased in lymphocytes from 2 of 5 AD DC subjects, whereas in the other 3 subjects Fas-mediated apoptosis was similar to that found in control cells. Taken together, these data suggest that the Fas signaling pathway is intact in AD DC lymphocytes and that other factors, likely related to diminished telomerase activity, may be contributing to the increased basal level of apoptosis.
In summary, AD DC is a unique human model system to study the effects of telomerase and shortened telomere length on a variety of cellular processes. The clinical observations of lymphopenia and hypogammaglobulinemia correlate well with the in vitro findings of diminished lymphocyte replication and increased apoptosis. Our data further substantiates the regulatory role of telomerase in B- and T-lymphocyte homeostasis and cellular function.
Prepublished online as Blood First Edition Paper, July 6, 2004; DOI 10.1182/blood-2004-04-1673.
Supported by the Carver Medical Trust Funds and grant no. IN-122Q from the American Cancer Society, administered through the University of Iowa Holden Comprehensive Cancer Center (F.G.). M.B. is supported from the American Cancer Society (ROG-03-104-01), National Institutes of Health (NIH), National Cancer Institute (NCI) CA105312, and the Bursary Award from the Aplastic Anemia & Myelodysplastic Syndromes (MDS) International Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank all the dedicated patients and their families who participated in this project and additionally Justine Ritchie for help with the statistical analyses and Al Klingelhutz for his critical review of the paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal