Yao et al1 reported the case of a girl with hemolytic anemia and severe rhabdomyolysis identified, with great ingenuity, as a compound heterozygote for 2 mutations in the ALDOA gene, namely the Arg303X “null allele,” and the Cys338Tyr substitution. Only 3 mutations are known for this gene,2-4 all occurred in patients with hemolytic anemia, in one case associated to myopathy.3
Given our long-standing interest in structure/function relationships in aldolase A and B proteins,5,6 we were prompted to examine the Cys338Tyr missense mutant, predicted to cause enzyme instability.1 Unexpectedly, the child's red cell aldolase activity was severely deficient, whereas plasma aldolase was normal. How can this be explained when both mutations severely affect enzyme function?
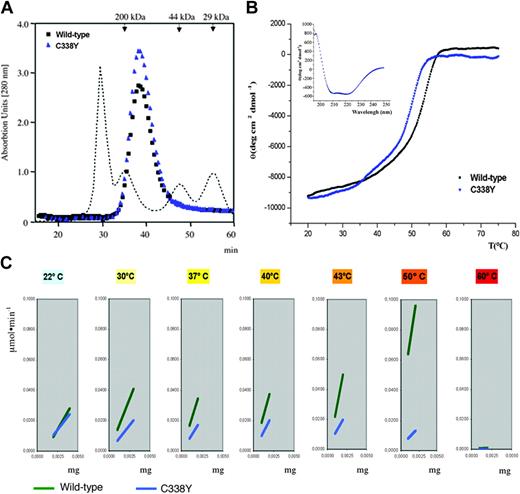
To address this issue, we synthesized the recombinant Cys338Tyr enzyme and conducted functional/structural analyses.4,5 Fast performance liquid chromatography (FPLC) and circular dichroism revealed a tetramer whose secondary structure matches that of the wild-type enzyme (Figure 1A,B [inset]). However, the melting temperature of Cys338Tyr was about 4°C lower (50.1°C versus 54.2°C; Figure 1B). At 30°C the overall catalytic rate (kcat) of Cys338Tyr was half that of the wild-type enzyme (4.5 ± 1.7 versus 9.2 ± 1.8 s-1), and Michaelis-Menten constant (Km) values were similar in the 2 enzymes (47.6 ± 6.5 versus 54.0 ± 5.4 μM). However, the activities of the enzymes diverged as temperature increased (Figure 1C). In agreement with thermal denaturation data, the Cys338Tyr mutant was almost inactive at 50°C, whereas the wild-type enzyme retained high activity. Neither enzyme was active at 60°C. Hence, the mutation-induced functional alterations are temperature dependent, which explains the patient's normal plasma aldolase activity.1 Indeed, the mutant enzyme becomes unstable only at temperatures beyond 30°C, as occurs during muscle exercise and fever.
Functional and structural features of recombinant wild-type and Cys338Tyr aldolase A. (A) FPLC profiles of the wild-type (black) and Cys338Tyr (blue) aldolase A enzymes. Both enzymes have a molecular mass of about 170 kDa, as expected for the histidine-tagged homo-tetrameric form of aldolase A. The dotted line and arrows indicate the molecular masses of the protein standards used in calibration, that is, β-amylase (200 kDa), chicken ovalbumin (44 kDa), and carbonic anhydrase (29 kDa) on a 50-cm by 1.6-cm Sephadex G-200 column (Amersham Biosciences, Milan, Italy) in 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.4), 20% (vol/vol) glycerol, with a flow rate of 0.2 mL/min. (B) Melting curves of wild-type (black) and Cys338Tyr (blue) aldolase A obtained by monitoring molar ellipticity at 222 nm. Inset: the CD spectra of wild-type (black) and Cys338Tyr (blue) aldolase A, recorded at 20°C with 0.4 mg/mL protein. (C) Specific activity represents μmol of hexose substrate cleaved × minute (μmol × minute-1) × mg of enzyme. The hexose fructose 1,6-biphosphate cleavage rate (μmol × minute-1) was determined spectrophotometrically by measuring nicotinamide adenine dinucleotide (NADH) oxidation at 340 nm in a coupled assay with α-glycerolphosphate dehydrogenase/triosophosphate isomerase.
Functional and structural features of recombinant wild-type and Cys338Tyr aldolase A. (A) FPLC profiles of the wild-type (black) and Cys338Tyr (blue) aldolase A enzymes. Both enzymes have a molecular mass of about 170 kDa, as expected for the histidine-tagged homo-tetrameric form of aldolase A. The dotted line and arrows indicate the molecular masses of the protein standards used in calibration, that is, β-amylase (200 kDa), chicken ovalbumin (44 kDa), and carbonic anhydrase (29 kDa) on a 50-cm by 1.6-cm Sephadex G-200 column (Amersham Biosciences, Milan, Italy) in 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.4), 20% (vol/vol) glycerol, with a flow rate of 0.2 mL/min. (B) Melting curves of wild-type (black) and Cys338Tyr (blue) aldolase A obtained by monitoring molar ellipticity at 222 nm. Inset: the CD spectra of wild-type (black) and Cys338Tyr (blue) aldolase A, recorded at 20°C with 0.4 mg/mL protein. (C) Specific activity represents μmol of hexose substrate cleaved × minute (μmol × minute-1) × mg of enzyme. The hexose fructose 1,6-biphosphate cleavage rate (μmol × minute-1) was determined spectrophotometrically by measuring nicotinamide adenine dinucleotide (NADH) oxidation at 340 nm in a coupled assay with α-glycerolphosphate dehydrogenase/triosophosphate isomerase.
We compared our functional data with molecular graphics data on the aldolase A structure (Protein Database code 1ALD) and its complexes with fructose 1,6-bisphosphate (4ALD) and dihydroxyacetone phosphate (1ADO). Cys338, the C-terminal residue of the α-helix 320 to 338, is not crucial for catalysis.6 Indeed, although conserved among aldolase A isoenzymes, Cys338 is frequently replaced with Ala and Ser in aldolases B and C. It is located in a tightly packed pocket formed by the main chain of Gly26-Leu27, Leu283, and Leu297-Thr298, all within 4.0 Å of Cys338 atoms. The replacement of Cys with a bulkier Tyr alters the structure of region 283 to 297, which includes Leu292 and Pro294 that contribute to tetramer assembly, thereby provoking local structural perturbation. At low temperatures, this perturbation does not affect enzyme activity, whereas at higher temperatures, local destabilization may propagate to other regions and affect enzyme activity and stability. These considerations coincide with the mutant's reduced thermostability.
In conclusion, even apparently minor alterations of the 3-dimensional structure of aldolase A can profoundly affect its in vivo activity, thereby explaining the severe phenotype reported by Yao and colleagues.1 Because aldolase A exerts a constitutive function in practically all human tissues, human embryos affected by severe ALDOA mutations do not survive. This would explain the rarity of ALDOA mutations versus ALDOB.7,8
Supported by Ministero dell'Instruzione, dell'Universitá e della Ricerca (DM 623/96; PS35-126/IND), Ministero della Salute (Progetti speciali, D.L.vo 299/99, bando 2001-02-03), and Regione Campania (Ricerca Sanitaria Finalizzata).
We thank Jean Ann Gilder for text editing.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal