Abstract
We tested the hypothesis that the antibody response to human factor IX in mice is controlled by genetic factors, especially histocompatibility antigens. Seven inbred mouse strains were immunized against human factor IX by adenoviral gene transfer or serial injections of human factor IX protein. A/J mice had the highest antibody response and 2 C57 mouse strains had the lowest response. We used the adenovirus vector to immunize 26 recombinant inbred mouse strains (AXB and BXA) derived from A/J and C57BL/6J mice and observed highly significant linkage (logarithmic odds [LOD] scores ∼4.8) for the polymorphic D17Mit62 marker that is 1 centimorgan (∼300 000 base pair [bp]) from the mouse major histocompatibility complex (MHC) locus (H-2). Experiments in mice with chimeric MHC genes indicated that class IaK or class II H-2 (or both) genes were critical, but other genes contributed to the antibody response. Polymorphic markers from chromosomes 1 and 10 that are near important immunoregulatory genes such as interleukin 10 and the interferon-γ gene show suggestive linkage (LOD scores of ∼2.3-2.6) to the factor IX antibody response. This study confirms the hypothesis that H-2 (and other) genes control factor IX antibody development in mice and suggests their potential importance for factor IX antibody development in humans with hemophilia B.
Introduction
Inhibitory antibodies to factor VIII or factor IX are currently the most serious adverse event associated with use of factor concentrates for treatment of hemophilia A or B. The lifetime cumulative incidence of inhibitor antibodies in hemophilia A is about 20% and in hemophilia B about 3%.1 The rates for patients with severe hemophilia A or B are even higher. The presence of an inhibitor antibody complicates factor replacement therapy by increasing the amount of factor VIII or factor IX needed to treat or prevent bleeding. When the titer is sufficiently high, replacement therapy is not practical and “bypassing” agents are required to treat bleeding; such treatment is not as satisfactory as factor VIII or factor IX replacement therapy. For bypassing agents no reliable in vitro tests predict adequate hemostasis, and high doses of these agents can cause thrombosis as an associated adverse event.
Inhibitor antibodies to factor VIII tend to be of the IgG4 and IgG1 subclasses in humans,2,3 and anaphylaxis or complications related to immune complex formation are rare even when patients with inhibitors are given human factor VIII protein. On the other hand, inhibitors to factor IX tend to be of the IgG1 subclass and anaphylaxis and renal complications attributable to immune complexes have been reported.4-7 Thus, although factor IX inhibitors are less common than factor VIII inhibitors, their clinical manifestations are more severe and the consequences to the patient are more serious than factor VIII inhibitors.
The tendency to form inhibitor antibodies may have a genetic as well as environmental basis, based on the observation that there is concordance within families with regard to inhibitor status and there may be differences in inhibitor frequency between racial groups.8-15 Data suggest that some HLA haplotypes in patients with hemophilia A may be associated with a greater likelihood of inhibitor development, but this relationship is not conclusive.16-18 As should be expected, deletions or large inversions of the factor VIII gene are associated with a higher incidence of inhibitors than other types of mutations in hemophilia A,18,19 and large deletions of the factor IX gene likewise are associated with a higher incidence of inhibitors in hemophilia B.20 Due to the lower incidence of hemophilia B and the less frequent occurrence of inhibitors to factor IX in hemophilia B, the role of HLA genes has not been studied as extensively as inhibitors to factor VIII in hemophilia A, and a causative role for genetics is even less certain in hemophilia B.
Mice with highly defined genetic content have been valuable tools for mapping genes associated with quantifiable phenotypes (“quantitative trait locus mapping”). The immune response to factor IX in mice has been observed to vary greatly; it has been observed empirically that C57BL/6J mice do not make high titer antibodies against human factor IX even when delivered to the liver by highly immunogenic adenovirus vectors,21 in contrast to most other strains of mice. We therefore decided to test the antibody response to human factor IX in mice to test the hypothesis that the antibody response to factor IX is controlled in part by genetic factors, especially histocompatibility loci. Our results indicate that in mice the major histocompatibility complex (MHC) locus is critical to the antibody response to human factor IX and that other genes also play a role.
Materials and methods
Animals
Inbred mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and maintained in the Food and Drug Administration (FDA) Center for Biologics Evaluation and Research vivarium. Animal protocols were approved by the FDA/Center for Biologics Evaluation and Research (CBER) Institutional Animal Care and Use Committee. Experiments using adenovirus vectors were performed under animal biohazard level 2 conditions.
Adenovirus vector AVC3FIX5T
Adenovirus vector AVC3FIX5T is an E1-, E3-deleted adenovirus serotype 5 vector that contains a human factor IX expression cassette under control of the cytomegalovirus (CMV) promoter22 and was prepared by repeated freeze-thaw lysis of infected HEK293 cells followed by ultracentrifugation in cesium chloride to purify the vector. Vector titers were determined by plaque assays on HEK293 cells and the number of vector particles was determined by measurement of optical density (OD) after disaggregation in sodium dodecyl sulfate (SDS)–Tris (tris(hydroxymethyl)aminomethane) buffer at 55°C.23 The vector used in this study was sterile and free of endotoxins or wild-type adenovirus.
Factor IX protein
Human factor IX protein used for immunization of mice and immunoassay for anti–human factor IX antibodies was sterile, clinical grade protein (Mononine; Aventis-Behring, Kankakee, IL).
Enzyme-linked immunosorbent assay for anti–human factor IX antibodies
Plates for antibody assays were coated with 0.1 μg/well Mononine (Aventis-Behring, Collegeville, PA) in 0.1 M sodium carbonate buffer (pH 9.2) and blocked with 6% bovine serum albumin (BSA) in phosphate-buffered saline (PBS)/0.05% Tween 20. Samples were diluted in blocking buffer and incubated overnight at 4°C. After washing with PBS/0.05% Tween 20, detector antibodies conjugated to horseradish peroxidase were applied for 1 hour at 37°C. Plates were washed and developed with 1 mg/mL o-phenylenediamine in 0.1 M sodium citrate (pH 4.5) then stopped with 1 M HCl. Titers were the greatest mean dilution of serum required to bring net absorbance down to that of the plate blank. Mouse monoclonal anti–human factor IX IgG (Boehringer-Mannheim, Indianapolis, IN) was used as a standard to convert absorbance values and sample dilutions to IgG levels in nanograms per milliliter. All assays were performed in duplicate.
ELISA for anti–adenovirus vector antibodies
Antibodies against adenovirus vector were determined by an enzyme-linked immunosorbent assay (ELISA) similar to that for anti–human factor IX antibodies except that plates were coated with 3.6 × 109 particles of vector AVC3FIX5T per well, instead of factor IX.
Statistical analysis of the mouse anti–human factor IX antibody response
One-way analysis of variance (ANOVA) for anti–human factor IX antibody levels was done using JMP version 4.04 statistical analysis software (SAS Institute, Cary, NC). A P value of less than .05 was required to conclude significant differences between groups being compared. Significance was further tested in the case of multiple comparisons or by the Dunnett correction for comparisons of multiple groups with a control group, as per JMP software.
Linkage analysis
Analysis of linkage of anti–human factor IX antibody titers with known polymorphisms in the mouse genome was performed with WebQTL software (Roswell Park Cancer Institute and the University of Tennessee Health Science Center, http://www.webqtl.org). The program calculates the association between a quantifiable trait (eg, antibody level) and any one or more of 753 polymorphisms that have been catalogued for recombinant inbred mouse lines in common use for gene mapping (eg, AXB strains and BXA strains).24 To test how robust the observed phenotypic data set is the program performs numerous analyses where the individual strain observations are randomly assigned weights of 0 to 1. The associations (linkage relatedness) that arise from these permutations of the data are used to establish a distribution of scores that define the significance of actual linkage relatedness scores that are obtained from unweighted data. In this study the default settings of 1000 permutations, 1000 bootstrap tests, and a minimum logarithmic odds (LOD) score cutoff of 2.0 were used for the linkage analysis.
Phage display analysis of antibody epitopes
Both 12-mer and 7-mer random peptide M13 phage display libraries were constructed according to the protocol of the New England Biolabs (Beverly, MA). The library contained approximately 109 unique clones. For the first round screening of libraries, phage (5 × 1010 plaque-forming unit [pfu] of each library) were adsorbed with the preimmune mouse sera in 50 mM Tris-Cl (pH 7.5), 150 mM NaCl, and 0.1% Tween-20. The unbound phage were then collected and further incubated with postimmune sera (1:100) and protein G–Sepharose beads (Amersham, Piscataway, NJ). The phage-antibody complexes were washed with Tris-buffered saline (TBS)–0.1% Tween-20 and phage were eluted with 0.1 N HCl, 0.2 M glycine, and 1 mg/mL BSA (pH 2.2). Selected phage were amplified in F+Escherichia coli strain ER2738 and purified by NaCl/polyethylene glycol (PEG) precipitation. In the second and third rounds of selection, approximately the same numbers of amplified phage (2 × 1010 pfu's) were incubated with the preimmune or postimmune immobilized antibodies using more stringent washing conditions. At the end of the third round of selection, 38 clones were randomly chosen, amplified, and sequenced.
Results
Inbred mouse strains differ in anti–human factor IX antibody levels in response to AVC3FIX5T
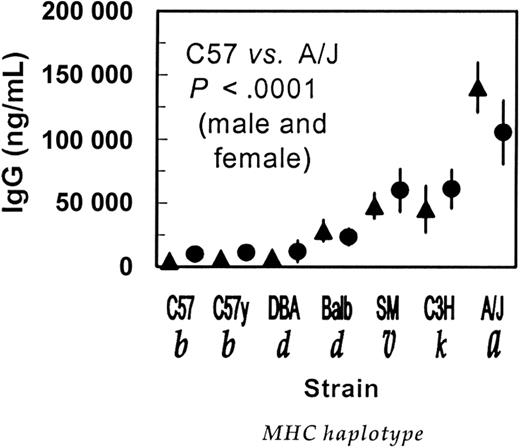
Males and females of 7 different inbred mouse strains were given 6.8 × 1011 vector particles/kg AVC3FIX5 vector, and their anti–human factor IX IgG antibody levels were measured 4 weeks later. IgG anti–human factor IX levels differed dramatically between strains and, to a lesser extent, between the sexes (Figure 1).
Anti–human factor IX IgG levels in inbred mouse strains differ following injection with AVC3FIX5 vector. Seven strains of inbred mice were injected with 6.8 × 1011 vector particles/kg AVC3FIX5 adenovirus vector and levels of anti–human FIX IgG were measured one month later. Ten males and 10 females from each strain were assessed. Mean IgG anti–human factor IX levels are shown with 95% confidence intervals (CIs) indicated by vertical lines (▴, males; •, females). As shown in the figure, the P values for comparison of antibody levels between C57 and A/J mice by one-way ANOVA were highly significant for both males and females (P < .0001 for either comparison). Pairwise comparisons of C57 mice with C3H, SM, and Balb mice were also statistically highly significant for both males and females (P < .001, not shown). The P values that were statistically significant retained their significance after adjustments by either the method of Dunnett for comparison of multiple groups to a control group (C57) or the Bonferroni correction to prevent type I error in multiple comparisons. Nonsignificant P values (> .45) were observed for one-way ANOVA comparisons of C57 with C57y or DBA mice. Strain abbreviations are C57 (C57BL/6J), C57y (C57BL/6ByJ), DBA (DBA/2J), Balb (Balb/cByJ), SM (SM/J), C3H (C3H/HeJ), and A/J (A/J). MHC H-2 haplotype designations are shown in italics below each strain.
Anti–human factor IX IgG levels in inbred mouse strains differ following injection with AVC3FIX5 vector. Seven strains of inbred mice were injected with 6.8 × 1011 vector particles/kg AVC3FIX5 adenovirus vector and levels of anti–human FIX IgG were measured one month later. Ten males and 10 females from each strain were assessed. Mean IgG anti–human factor IX levels are shown with 95% confidence intervals (CIs) indicated by vertical lines (▴, males; •, females). As shown in the figure, the P values for comparison of antibody levels between C57 and A/J mice by one-way ANOVA were highly significant for both males and females (P < .0001 for either comparison). Pairwise comparisons of C57 mice with C3H, SM, and Balb mice were also statistically highly significant for both males and females (P < .001, not shown). The P values that were statistically significant retained their significance after adjustments by either the method of Dunnett for comparison of multiple groups to a control group (C57) or the Bonferroni correction to prevent type I error in multiple comparisons. Nonsignificant P values (> .45) were observed for one-way ANOVA comparisons of C57 with C57y or DBA mice. Strain abbreviations are C57 (C57BL/6J), C57y (C57BL/6ByJ), DBA (DBA/2J), Balb (Balb/cByJ), SM (SM/J), C3H (C3H/HeJ), and A/J (A/J). MHC H-2 haplotype designations are shown in italics below each strain.
C57BL/6J and the near-identical C57BL/6ByJ strain had virtually no anti–human factor IX IgG antibodies after adenovirus vector administration. The highest levels of anti–human factor IX IgG antibodies were demonstrated in A/J mice, whose levels were more than 20 times higher than those in C57BL/6J mice (P < .0001). Intermediate levels of anti–human factor IX IgG were found in DBA/2J, Balb/cByJ, SM/J, and C3H/HeJ mice. For most mice, there was a tendency toward higher antibody levels in females of a strain as compared to males; however, in A/J mice this tendency was reversed, with higher titers observed in the males. The 2 low-titer C57BL/6 strains (C57BL/6J and C57BL/6ByJ) have the MHC H-2 “b” haplotypes, whereas the high-responding A/J strain has the “a” haplotype, which is actually a chimera of “k” and “d” mouse MHC H-2 haplotypes (Table 1).
Mouse strain MHC H-2 haplotypes and genes
Strain (haplotype) . | K class I-a . | Aβ class II . | Aα class II . | Eβ class II . | Eα class II . | S class III . | D class I-a . | L class I-a . | Qa-2 class I-b . | T18 class I-b . |
|---|---|---|---|---|---|---|---|---|---|---|
| A/J (a) | k | k | k | k | k | d | d | d | a | a |
| C3H/HeJ (k) | k | k | k | k | k | k | k | k | b | b |
| SM/J (v) | v | v | v | v | v | v | v | v | a | b |
| Balb/cByJ (d) | d | d | d | d | d | d | d | d | b | d |
| DBA/2J (d) | d | d | d | d | d | d | d | d | b | d |
| C57BL/5ByJ (b) | b | b | b | — | b | b | b | — | a | b |
| C57BL/6J (b) | b | b | b | — | b | b | b | — | a | b |
Strain (haplotype) . | K class I-a . | Aβ class II . | Aα class II . | Eβ class II . | Eα class II . | S class III . | D class I-a . | L class I-a . | Qa-2 class I-b . | T18 class I-b . |
|---|---|---|---|---|---|---|---|---|---|---|
| A/J (a) | k | k | k | k | k | d | d | d | a | a |
| C3H/HeJ (k) | k | k | k | k | k | k | k | k | b | b |
| SM/J (v) | v | v | v | v | v | v | v | v | a | b |
| Balb/cByJ (d) | d | d | d | d | d | d | d | d | b | d |
| DBA/2J (d) | d | d | d | d | d | d | d | d | b | d |
| C57BL/5ByJ (b) | b | b | b | — | b | b | b | — | a | b |
| C57BL/6J (b) | b | b | b | — | b | b | b | — | a | b |
Mouse strains are listed with the MHC H-2 haplotype designation in italics, along with the allele designation for each MHC H-2 complex gene. — indicates that the genetic material at a locus is absent from the C57BL/6J strain.
Although the C57BL/6J mice were the lowest responders, the IgG anti–human factor IX that they did produce was predominantly of the IgG1 subclass, as was the IgG anti–human factor IX found in A/J mice (data not shown). Thus, the difference in the antibody levels was not associated with shifts of IgG isotype.
There is the possibility that the difference in the antibody response in A/J and C57BL/6J mice was due to differences in the underlying mouse factor IX protein sequence, that is, that there were more “foreign” human factor IX protein epitopes for one strain (A/J) than for the other (C57BL/6J). We therefore sequenced the cDNAs for factor IX protein derived from liver mRNA in A/J and C57BL/6J mice to rule out differences between endogenous mouse factor IX in these strains. Both were identical to the mouse factor IX cDNA sequence published by Wu et al.25
Human factor IX protein in the absence of adjuvant induces antibodies in mice, albeit at lower levels than adenovirus vector AVC3FIX5T
To test the hypothesis that the differential antibody response is peculiar to presentation by adenovirus vector, we injected A/J and C57BL/6J mice with human factor IX protein (Mononine) at a dose of 50 U/kg, on alternating days for 14 days (7 intravenous injections, without adjuvant). Although antibody levels in both strains 1 month after the beginning of protein injections were much lower than with adenovirus vector administration, the antibody response in A/J mice was greater than that of C57BL/6J (P = .002 for males, P = .0037 for females; Table 2). In fact, the response of C57BL/6J mice after administration of Mononine was essentially undetectable. This indicates that the higher response of A/J mice to human factor IX (compared to C57BL/6J mice) is a function of the interaction between the immune system and the protein rather than a function of expression from an adenovirus vector. The much higher titers following gene transfer, compared to levels following exposure to human factor IX protein itself, also indicate the strong adjuvant effect of the adenovirus vector. As was true for adenovirus vector-induced anti–human factor IX antibodies, the antibody response to human factor IX protein injections was predominantly of the IgG1 isotype (data not shown).
Anti–human factor IX IgG levels following injection of human factor IX protein
Group . | No. . | Mean IgG anti—human factor IX, ng/mL . | SD . |
|---|---|---|---|
| A/J males | 10 | 5300 | 4721 |
| A/J females | 9 | 1727 | 1716 |
| C57BL/6J males | 10 | 0 | 0 |
| C57BL/6J females | 10 | 15 | 48 |
Group . | No. . | Mean IgG anti—human factor IX, ng/mL . | SD . |
|---|---|---|---|
| A/J males | 10 | 5300 | 4721 |
| A/J females | 9 | 1727 | 1716 |
| C57BL/6J males | 10 | 0 | 0 |
| C57BL/6J females | 10 | 15 | 48 |
On days 1, 3, 5, 7, 9, 11, and 13, 50 U/kg was given intravenously; antibody levels were measured 1 month after first protein injection. Values are means of anti—human factor IX levels, calculated using a standard curve of monoclonal anti—human factor IX antibody at defined concentrations. P = .002 for A/J male versus C57BL/6J male; P = .0037 for A/J female versus C57BL/6J female.
Antiadenovirus titers are similar in A/J and C57BL/6J mice
Antiadenovirus antibody titers in A/J and C57BL/6J mice 1 month after vector injection are high in both strains (in fact somewhat higher in C57BL/6J female mice than in A/J female mice) indicating that the low response to human factor IX protein in C57BL/6J is not due to inability to produce antibodies to foreign proteins by C57BL/6J mice (Table 3). The differences between strains did not reach statistical significance (P = .52 for males, P = .09 for females).
Anti–AVC3FIX5 IgG titers following intravenous injection of AVC3FIX5 vector
Group . | No. . | Mean IgG anti-AVC3FIX5 titer . | SD . |
|---|---|---|---|
| A/J males | 10 | 92 160 | 21 578 |
| A/J females | 10 | 66 560 | 24 732 |
| C57BL/6J males | 10 | 84 480 | 29 683 |
| C57BL/6J females | 10 | 102 400 | 59 121 |
Group . | No. . | Mean IgG anti-AVC3FIX5 titer . | SD . |
|---|---|---|---|
| A/J males | 10 | 92 160 | 21 578 |
| A/J females | 10 | 66 560 | 24 732 |
| C57BL/6J males | 10 | 84 480 | 29 683 |
| C57BL/6J females | 10 | 102 400 | 59 121 |
The injection contained 6.8 × 1011 vector particles/kg. Values are means of reciprocals of greatest dilutions of mouse sera required to bring net OD 492 equal to that of the plate blank. There was no statistically significant difference between A/J males and C57BL/6J males (P = .52), or for A/J females versus C57BL/6J females (P = .09).
Response of B10.A congenic mice to AVC3FIX5
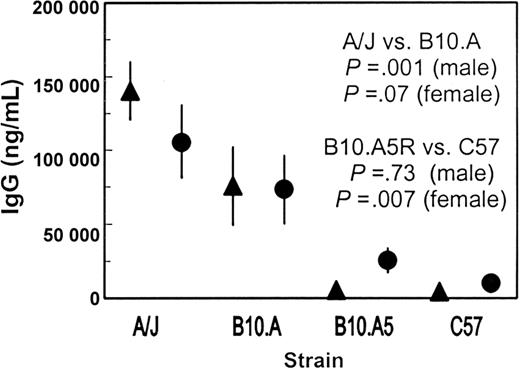
C57BL/6J and C57BL/10J are virtually identical substrains of the C57BL mouse line derived at the Jackson Laboratories during the 1920s. B10.A-H2a H2-T18a/SgSnJ mice (B10.A) were derived by breeding C57BL/10J mice with A/J mice, then repeatedly breeding back to C57BL/10J mice while selecting progeny having only the MHC H-2 genes from the A/J strain. These mice have the A/J MHC H-2 genes in a C57BL background and present an excellent experimental system in which to test the effect of A/J MHC H-2 genes to the anti–human factor IX response in a strain essentially identical to the nonresponsive C57BL/6J strain. B10.A mice have an antibody response about 50% that of A/J mice (Figure 2 and Table 4). The difference between A/J and B10.A is highly significant for males (P = .001) but is not quite significant for females (P = .07).
Anti–human factor IX IgG levels in B10.A congenic mice following intravenous injection of AVC3FIX5 vector indicate role of MHC H-2 genes. B10.A-H2aH2-T18a/SgSnJ and B10.A-H2i5H2-T18a(5R)/SgSnJ mice were injected with 6.8 × 1011 vector particles/kg AVC3FIX5 adenovirus vector and IgG levels versus human factor IX were measured 1 month later. Ten males and 10 females from each strain were assessed. Mean IgG anti–human factor IX levels are shown with 95% CIs indicated by vertical lines (▴, males; •, females). Mouse strains are A/J (A/J), B10.A-H2aH2-T18a/SgSnJ (B10.A), B10.A-H2i5H2-T18a(5R)/SgSnJ (B10.A5R), and C57BL/6J (C57).
Anti–human factor IX IgG levels in B10.A congenic mice following intravenous injection of AVC3FIX5 vector indicate role of MHC H-2 genes. B10.A-H2aH2-T18a/SgSnJ and B10.A-H2i5H2-T18a(5R)/SgSnJ mice were injected with 6.8 × 1011 vector particles/kg AVC3FIX5 adenovirus vector and IgG levels versus human factor IX were measured 1 month later. Ten males and 10 females from each strain were assessed. Mean IgG anti–human factor IX levels are shown with 95% CIs indicated by vertical lines (▴, males; •, females). Mouse strains are A/J (A/J), B10.A-H2aH2-T18a/SgSnJ (B10.A), B10.A-H2i5H2-T18a(5R)/SgSnJ (B10.A5R), and C57BL/6J (C57).
Anti–human factor IX IgG levels in B10.A congenic mice following intravenous injection of AVC3FIX5 vector indicate role of MHC H-2 genes
. | MHC H-2 genes . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain . | K class I-a . | Aβ class II . | Aα class II . | Eβ class II . | Eα class II . | S class III . | D class I-a . | L class I-a . | Qa-2 class I-b . | T18 class I-b . | |||||||||
| A/J | k | k | k | k | k | d | d | d | a | a | |||||||||
| B10.A | k | k | k | k | k | d | d | d | a | a | |||||||||
| B10.A5R | b | b | b | b/k | k | d | d | d | a | a | |||||||||
| C57BL/6J | b | b | b | — | b | b | b | — | a | b | |||||||||
. | MHC H-2 genes . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain . | K class I-a . | Aβ class II . | Aα class II . | Eβ class II . | Eα class II . | S class III . | D class I-a . | L class I-a . | Qa-2 class I-b . | T18 class I-b . | |||||||||
| A/J | k | k | k | k | k | d | d | d | a | a | |||||||||
| B10.A | k | k | k | k | k | d | d | d | a | a | |||||||||
| B10.A5R | b | b | b | b/k | k | d | d | d | a | a | |||||||||
| C57BL/6J | b | b | b | — | b | b | b | — | a | b | |||||||||
Mouse strains are A/J (A/J), B10.A-H2a H2-T18a/SgSnJ (B10.A), B10.A-H2i5 H2-T18a (5R)/SgSnJ (B10.A5R), and C57BL/6J (C57). — indicates that the genetic material at a locus is absent from the C57BL/6J strain.
B10.A-H2i5H2-T18a(5R)/SgSnJ mice (B10.A5R) were derived from the same breeding strategy that produced B10.A mice; however, they have part of the MHC H-2 complex from A/J but the same H-2 class Ia and class II genes as C57BL/10J mice. They exhibited a low antibody response to human factor IX, equal to that of C57BL/6J in the males (P = .73) but higher than C57BL/6J for the females (P = .007; Figure 2 and Table 4). These data indicate that most of the high antibody response of A/J mice is attributable to the contribution of their MHC H-2 genes in males, particularly the class Ia or class II genes (or both). Because there are no B10.A congenic strains having only the class II genes from A/J present in the C57BL/10J background, it is not possible to discern from these experiments whether the class II genes alone mediate the MHC H-2–dependent contribution of the antibody response in A/J mice. However, the fact that injections of human factor IX protein alone (which would be presented as any exogenous antigen to the immune system by the class II pathway) elicited antibodies in A/J mice but not in C57BL/6J mice suggests that the critical genetic determinant is the class II gene cluster in the MHC H-2 locus.
Response of AXB and BXA recombinant inbred mice to AVC3FIX5
Recombinant inbred (RI) mice are derived by breeding of F1 progeny of a cross between 2 different parental strains, then inbreeding between different brother-sister pairs among the F2 progeny. After extensive inbreeding of different F2 sib pairs (at least 20 generations), multiple congenic strains are derived in which half of the genetic content is derived from each original parental strain. These strains differ one from another in the random distribution of the parental strain alleles, so measurement of a quantitative trait in multiple RI lines derived from parental strains that differ in the trait in question can be used to determine an association of the trait with genetic polymorphisms that differ between strains, where these are known.26
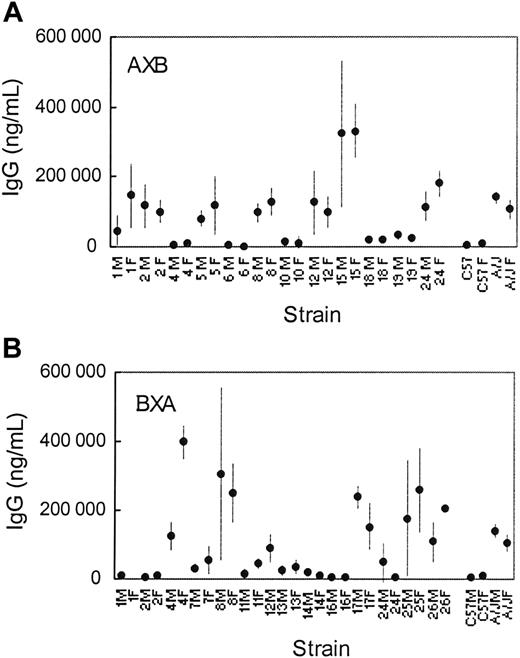
Fortuitously, a series of RI mice, known as AXB (where the initial mating is between an A/J female and a C57BL/6J male) or BXA (where the initial mating is the reverse of AXB) have been derived from A/J and C57BL/6J parental strains and more than 753 polymorphic sites have been cataloged throughout the gDNA of each strain.24 AXB and BXA RI mouse strains that had been inbred for 60 to 100 generations were obtained from the Jackson Laboratories and immunized using the AVC3FIX5 vector and the antibody response to human factor IX was assessed in each strain. The data (Figure 3) permit calculation of the association of the anti–human factor IX antibody with polymorphic markers that differ between A/J and C57BL/6J mice, using quantitative trait analysis software by WebQTL (http://www.webqtl.org).
Mouse IgG anti–human factor IX following AVC3FIX5 as a function of RI mouse strain. (Top) AXB RI mouse strains. (Bottom) BXA RI mouse strains. Mean IgG anti–human factor IX levels are shown with 95% CIs indicated by vertical lines for each panel. M indicates male; F, female. A/J and C57BL/6J antibody levels (from Figure 1) are also shown in each panel for reference purposes.
Mouse IgG anti–human factor IX following AVC3FIX5 as a function of RI mouse strain. (Top) AXB RI mouse strains. (Bottom) BXA RI mouse strains. Mean IgG anti–human factor IX levels are shown with 95% CIs indicated by vertical lines for each panel. M indicates male; F, female. A/J and C57BL/6J antibody levels (from Figure 1) are also shown in each panel for reference purposes.
The most significant associations of anti–human factor IX antibody formation with polymorphic markers that were determined from the analyses of AXB and BXA recombinant mice are listed in Table 5. The most significant association was with the marker D17Mit62. This marker is less than 1 centimorgan (cM; ∼300 000 bp) from the MHC H-2 locus (Figure 4), as would be predicted from the prior analysis of the various inbred mouse strains, B10.A congenic strains, and injection of A/J and C57BL/6J mice with human factor IX protein. The LOD score for the association between D17Mit62 and anti–human factor IX antibodies is highly significant for both male and female mice (LOD scores of ∼4.8 for both).
Linkage scores for polymorphic markers in male mice associated with IgG response to human factor IX following AVC3FIX5 administration
LRS (LOD) . | Chromosome . | Marker . |
|---|---|---|
| 21.994 (LOD 4.8) | 17 | D 17Mit 62 |
| 12.202 (LOD 2.6) | 1 | D 1Mit 100 |
| 12.202 (LOD 2.6) | 1 | D 1Mit 264 |
| 12.202 (LOD 2.6) | 1 | D 1Mit 199 |
| 11.994 (LOD 2.6) | 10 | D 10Mit 179 |
| 10.518 (LOD 2.3) | 10 | D 10Mit 74 |
| 10.518 (LOD 2.3) | 10 | D 10Mit 237 |
| 10.518 (LOD 2.3) | 10 | D 10Mit 267 |
| 10.518 (LOD 2.3) | 10 | D 10Mit 180 |
LRS (LOD) . | Chromosome . | Marker . |
|---|---|---|
| 21.994 (LOD 4.8) | 17 | D 17Mit 62 |
| 12.202 (LOD 2.6) | 1 | D 1Mit 100 |
| 12.202 (LOD 2.6) | 1 | D 1Mit 264 |
| 12.202 (LOD 2.6) | 1 | D 1Mit 199 |
| 11.994 (LOD 2.6) | 10 | D 10Mit 179 |
| 10.518 (LOD 2.3) | 10 | D 10Mit 74 |
| 10.518 (LOD 2.3) | 10 | D 10Mit 237 |
| 10.518 (LOD 2.3) | 10 | D 10Mit 267 |
| 10.518 (LOD 2.3) | 10 | D 10Mit 180 |
The polymorphic marker D17mit62 is near the mouse MHC H-2 gene cluster on chromosome 17. D17Mit62 is the polymorphic marker with the highest association with anti–human factor IX antibody levels in AXB and BXA recombinant inbred mouse strains. Mouse chromosome 17 is depicted with a horizontal line. Polymorphic markers that were analyzed for their association with antibody development are shown above the line. The map positions along chromosome 17 (in centimorgans [cM] or megabases [Mb]) are shown below the line, as are genes of the histocompatibility complex H-2 locus.
The polymorphic marker D17mit62 is near the mouse MHC H-2 gene cluster on chromosome 17. D17Mit62 is the polymorphic marker with the highest association with anti–human factor IX antibody levels in AXB and BXA recombinant inbred mouse strains. Mouse chromosome 17 is depicted with a horizontal line. Polymorphic markers that were analyzed for their association with antibody development are shown above the line. The map positions along chromosome 17 (in centimorgans [cM] or megabases [Mb]) are shown below the line, as are genes of the histocompatibility complex H-2 locus.
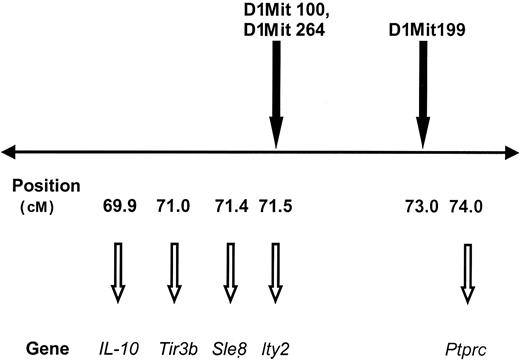
The antibody response of mice with A/J MHC H-2 genes in the C57BL/10J background is about half of that seen in A/J mice, suggesting that there are additional genes that increase the antibody response to human factor IX aside from the MHC H-2 genes. A series of polymorphic markers on mouse chromosomes 1 and 10 demonstrate suggestive associations with anti–human factor IX antibodies, as shown in Table 5. Three markers on chromosome 1 (D1Mit100, D1Mit199, and D1Mit264) have LOD scores of about 2.6 and are near genes with important roles in immunity, including interleukin 10 (IL-10) and CD45 (Figure 5).
The polymorphic markers D1Mit100, D1Mit264, and D1Mit199 are near various immunoregulatory genes on chromosome 1. Mouse chromosome 1 is depicted with a horizontal line. Polymorphic markers associated with antibody development are shown above the line. The map positions along chromosome 1 (in cM) are shown below the line, as are genes found near these markers. Tir3b indicates trypanosomiasis infection response gene 3b; Sle8, systemic lupus erythematosus susceptibility gene 8; Ity2, immunity to Salmonella typhimurium 2 gene; Ptprc, protein tyrosine phosphatase, receptor type, C (also called CD45, B220).
The polymorphic markers D1Mit100, D1Mit264, and D1Mit199 are near various immunoregulatory genes on chromosome 1. Mouse chromosome 1 is depicted with a horizontal line. Polymorphic markers associated with antibody development are shown above the line. The map positions along chromosome 1 (in cM) are shown below the line, as are genes found near these markers. Tir3b indicates trypanosomiasis infection response gene 3b; Sle8, systemic lupus erythematosus susceptibility gene 8; Ity2, immunity to Salmonella typhimurium 2 gene; Ptprc, protein tyrosine phosphatase, receptor type, C (also called CD45, B220).
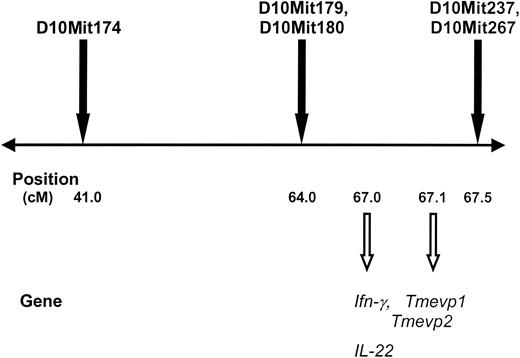
Five markers on mouse chromosome 10 (D10Mit179, D10Mit180, D10Mit74, D10Mit237, and D10Mit267) with suggestive LOD scores (2.3-2.6) are near critical immunoregulatory genes as shown in Figure 6.
The polymorphic markers D10Mit174, D10Mit179, D10Mit180, D10Mit237, and D10Mit267 are near the mouse IFNγ gene on chromosome 10. Mouse chromosome 10 is depicted with a horizontal line. Polymorphic markers that were analyzed for their association with antibody development are shown above the line. The map positions along chromosome 10 (in cM) are shown below the line, as are genes located near the associated markers. Tmevp1 and 2 indicate Theiler murine encephalomyelitis virus persistence genes 1 and 2.
The polymorphic markers D10Mit174, D10Mit179, D10Mit180, D10Mit237, and D10Mit267 are near the mouse IFNγ gene on chromosome 10. Mouse chromosome 10 is depicted with a horizontal line. Polymorphic markers that were analyzed for their association with antibody development are shown above the line. The map positions along chromosome 10 (in cM) are shown below the line, as are genes located near the associated markers. Tmevp1 and 2 indicate Theiler murine encephalomyelitis virus persistence genes 1 and 2.
Two of these markers with LOD scores of 2.3 (D10Mit237 and D10Mit267) are less than half a centimorgan from the mouse interferon-γ (IFN-γ) gene and related genes that regulate IFN-γ expression. IFN-γ is a cytokine that is critical to control of the immune response and is thought to predispose mice toward TH2 rather than TH2 immune responses for some antigens. The former is predominantly a cellular response and the latter is predominantly a humoral response, which could in part help to explain the relatively low antibody response to human factor IX in C57BL/6J mice.
Epitopes in human factor IX that react with mouse antibodies
M13 phage libraries displaying random 7-mer and 12-mer amino acid sequences were tested for binding to antibodies in pooled A/J mouse serum from animals immunized with Mononine. Thirty-four phage sequences could be matched to human factor IX sequence. Of these, 15 clone sequences showed homology to human factor IX in the activation peptide region (Ile164 to Asn176), 7 had homology to sequence in the second epidermal growth factor–like domain (Val135 to Thr140), and the remainder were homologous to sequences in the catalytic domain (Leu322 to Leu329 and Tyr398 to Tyr404).
Discussion
The availability of inbred animals that are extremely well characterized with regard to genetic variation permits systematic study of the genetics of the immune response to clotting factor proteins. In contrast to other strains, C57BL/6J mice usually do not make antibodies to human factor IX following systemic or hepatic gene transfer, even when viral vectors with strong adjuvant properties (eg, adenovirus) are used.21 This empiric observation presented the opportunity to dissect the genetic factors that control the immune response to human factor IX in mice. The results of our study indicate that the MHC H-2 (class II) genes are the most critical genetic determinants of the antibody response to human factor IX protein in mice. Although this is not surprising, it is notable that a previous study of the antibody response to α1-antitrypsin delivered to the liver by adenovirus gene transfer showed no correlation with the MHC H-2 haplotype in various inbred strains of mice.27 The MHC H-2 locus does not completely account for the genetically controlled influence on antibody development because the high responder MHC H-2 genes in a low responder background (B10.A) are associated with only about half the antibody response as the high responder A/J mouse (Table 1). Therefore, other genes have additional influence over the level of antibodies that are seen in A/J mice (particularly males). Linkage has now been established between the antibody response and polymorphic markers near other candidate genes that control immunity (Table 5). Plausible candidate genes include those for IL-10 and IL-22, and IFN-γ, among others (Figures 5, 6). IL-10 is produced by TH2 CD4+ T lymphocytes (and other cells) and promotes antibody production by increasing B-cell activation. Additionally, it down-regulates the production of IFN-γ. IL-22 is homologous to IL-10 but does not stimulate antibody production by activated B lymphocytes.28 IFN-γ is produced by TH1 CD4+ T lymphocytes and enhances antigen presentation by antigen-presenting cells and macrophages. CD4+ T lymphocytes recognize antigens that are presented in the context of class II MHC molecules and, therefore, are critically important to the process of antibody production against factor IX. The C57BL/6J mouse strain is predisposed to the TH1 rather than TH2 immune response pattern for some antigens.29 Both C57BL/6J and A/J mice make high-titer antibodies to adenovirus vector (Table 3), indicating that the lack of antibody to human factor IX protein in C57BL/6J mice is not due to a general inability to make antibodies to proteins.
Knockout mice in the C57BL/6J background that were deficient in either IL-10 or IFN-γ were tested for their response to human factor IX and had no greater antibody levels than the C57BL/6J strain (data not shown). Thus, these genes probably have their main effect on the antibody response of A/J mice and may promote the higher antibody response that was observed in this strain. Because A/J IL-10 or IFN-γ knockout mice are not available, we are unable to test this hypothesis.
One potential shortcoming of our study is that the presence of (mouse) factor IX may skew the distribution of epitopes that are recognized as “foreign” by the immunized mice. Although it was not our primary interest to map the epitopes that are recognized by mouse anti–human factor IX antibodies, it is interesting that the most frequently recognized epitope in A/J mice immunized with factor IX overlapped with activation peptide epitopes that are recognized by inhibitor antibodies from 3 different patients with hemophilia B.30-32 Other reactive epitopes were found near the activation peptide in the second EFG-like domain, and others were near the carboxyl terminus of the protein in the catalytic domain. Antibodies to factor IX have also been mapped to the catalytic domain in patients with hemophilia B with inhibitors.32 Thus, despite its limitations, our study in normal mice correlates with human hemophilia B.
It is possible (even likely) that if factor IX knockout mice were available in the AXB/BXA recombinant inbred mice the antibody response in mice with genes from the high-responder (A/J) parental strain would be even higher than that which we saw in mice with normal factor IX levels. However, such mice are not available and it would take years to develop congenic AXB or BXA hemophilia B mice by breeding existing factor IX knockout mice onto these backgrounds.
The demonstration that multiple genes influence production of antibodies to human factor IX in mice suggests it may be useful to study these genes in patients with hemophilia B to see if they influence inhibitor antibody development. As an intermediate step it may be worthwhile to extend our studies to other animals such as dogs with hemophilia B, to approximate the human factor IX antibody response in a relatively outbred species. Our understanding of the genetic variation in dogs is much less comprehensive than in mice, so our ability to analyze the genetics of the immune response to factor IX in dogs is somewhat limited. Variation in dog leukocyte antigen complex class I and II genes has been described and analyzed serologically and at the molecular level,33 so it should be possible to at least study the effect of class II genes on development of anti–canine factor IX antibodies in dogs with hemophilia B.
Lastly, this study offers an explanation for the lack of responsiveness of C57BL/6J mice to human factor IX that has been exploited to permit testing of gene transfer vectors that otherwise might be expected to make antibodies to human factor IX in other inbred mouse strains.
Prepublished online as Blood First Edition Paper, September 21, 2004; DOI 10.1182/blood-2004-03-1126.
Presented in part at the 45th Annual Meeting of the American Society of Hematology, San Diego, CA, December 8, 2003. This is a US Government work; there is no restriction on its use.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr David Bodine for suggestions on use of recombinant inbred mouse strains and Dr David Scott for advice on the use of B10.A congenic mouse strains to evaluate the contribution of MHC H-2 genes to the immune response. We also thank Dr Jessica Kim for advice on the statistical methods for analysis of variance between mouse strains.

![Figure 4. The polymorphic marker D17mit62 is near the mouse MHC H-2 gene cluster on chromosome 17. D17Mit62 is the polymorphic marker with the highest association with anti–human factor IX antibody levels in AXB and BXA recombinant inbred mouse strains. Mouse chromosome 17 is depicted with a horizontal line. Polymorphic markers that were analyzed for their association with antibody development are shown above the line. The map positions along chromosome 17 (in centimorgans [cM] or megabases [Mb]) are shown below the line, as are genes of the histocompatibility complex H-2 locus.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-03-1126/6/m_zh80030573580004.jpeg?Expires=1767738954&Signature=GcEVRS8wVTCcqm720qmui8BjDbP~gQHfS9O-1XWeFnFEZAjFeq4vQ~eUsos29NtpJykNqi6QP0fvVVPdKsdd4Hho5rHuxqvxm1a6lyWReDrPHS9GajDfJ1SlcWtL9W~Fyd21D5yYmzWRsUi9LjzW4FkeG3eqV3Kw~L1OguCGSsf6D8-RzGzLQaKfnyIafTjuchtDQbkS31TbrtkLON43lRyLcQBQm-L8HBTxMH72QfzZO8CxQ0SweB6y4bjUbvfDFD51xqW~a8WNyZGkfDYZRh5Zt4jRTZLijdin6slZgWu5A5zE1erIFa~WmzQt~tmd-Y-5m~kaT8DQ228AY2wrlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal