Abstract
Gemtuzumab ozogamicin (GO; Mylotarg), a novel immunoconjugate used for treatment of acute myeloid leukemia (AML), contains the humanized anti-CD33 antibody (hP67.6) as a carrier to facilitate cellular uptake of the toxic calicheamicin-γ1 derivative. By use of lentivirus-mediated gene transfer to manipulate CD33 expression in myeloid cell lines that normally lack CD33 (murine 32D cells) or have very low levels of CD33 (human OCI-AML3 and KG-1a cells), we here show a quantitative relationship between CD33 expression and GO-induced cytotoxicity. The CD33 cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIMs) control internalization of antibody bound to CD33. Disruption of the ITIMs by introduction of point mutations not only prevented effective internalization of antibody-bound CD33 but also significantly reduced GO-induced cytotoxicity. Together, our data imply a pivotal role of both the number of CD33 molecules expressed on the cell surface and the amount of internalization of CD33 following antibody binding for GO-induced cytotoxicity and suggest novel therapeutic approaches for improvement of clinical outcome of patients treated with GO.
Introduction
CD33 is a 67 kDa type 1 transmembrane sialoglycoprotein and founding member of a rapidly evolving immunoglobulin superfamily subset of sialic acid–binding immunoglobulin-related lectins (siglecs; siglec-3).1,2 CD33 is characterized by the presence of 2 conserved immunoreceptor tyrosine-based inhibitory motif (ITIM)–like motifs in the cytoplasmic region, a property that is shared with all CD33-related siglecs discovered so far.2 If phosphorylated upon treatment with pervanadate or anti-CD33 antibody plus cross-linking secondary antibody, these tyrosine motifs recruit and activate Src homology-2 (SH2) domain-containing tyrosine phosphatases (SHP-1 and SHP-2).3-5
Physiologically, CD33 expression is restricted to early multilineage hematopoietic progenitors, myelomonocytic precursors, and more mature myeloid cells, but it is absent on normal pluripotent hematopoietic stem cells.6-8 However, 85% to 90% of adult and pediatric cases of acute myeloid leukemia (AML) express CD33.8,9 Therefore, CD33 has gained clinical importance as a suitable tumor-associated antigen and target for antibody-based AML therapies.10
Promising clinical results have been obtained with gemtuzumab ozogamicin (GO; CMA-676; Mylotarg; Wyeth Pharmaceuticals, Philadelphia, PA), an immunoconjugate consisting of a humanized immunoglobulin G4 (IgG4) anti-CD33 monoclonal antibody (hP67.6) joined to a derivative of the cytotoxic drug calicheamicin-γ1, N-acetyl-γ-calicheamicin dimethyl hydrazide.11 Although some unconjugated anti-CD33 antibodies may have limited cytoreductive activity when used as monotherapy in patients,12,13 hP67.6 is believed to function primarily as a carrier to facilitate cellular uptake of the calicheamicin-γ1 derivative, which is then cleaved intracellularly in an acid compartment, presumably inside lysosomes, and subsequently induces DNA damage and eventually cell death.14
This putative mechanism of action of GO still needs firm experimental confirmation but implies a pivotal role of both the number of CD33 molecules expressed on the cell surface and the rate of internalization of CD33 following GO binding for the cytotoxic effect. However, little is known about the importance of the quantity of CD33 expression for GO-induced cytotoxicity. Although multiple studies have confirmed that binding of bivalent and multivalent anti-CD33 antibodies results in CD33 internalization in both CD33+ hematopoietic cell lines and primary AML blast cells,15-18 some authors have argued that cytotoxic effects of GO are achieved in the absence of CD33 expression.19 In addition, neither the endocytic process by which CD33 delivers antibody to the cytosol nor the necessity of CD33 endocytosis for GO-induced cytotoxicity has been established.
The primary objective of the present study was therefore to investigate the quantitative relationship between CD33 expression and the requirement for CD33 internalization in GO-mediated cytotoxicity. To accomplish this, lentivirus-mediated gene transfer was used to manipulate CD33 expression. We also identified the motifs that control internalization of antibody-bound CD33 and determined the requirement of these motifs for GO-induced cytotoxicity.
Materials and methods
Cell lines
Human myeloid OCI-AML3 cells (kindly provided by Dr M. D. Minden, University of Toronto, ON) and KG-1a cells (kindly provided by Dr D. E. Banker, Fred Hutchinson Cancer Research Center, Seattle, WA) were maintained in RPMI medium 1640 with 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (GIBCO Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT), 1 mM minimum essential media (MEM) sodium pyruvate, and 0.1 mM MEM nonessential amino acids (both GIBCO Invitrogen). The interleukin-3 (IL-3)–dependent murine myeloid 32D cell line was maintained in Iscove modified Dulbecco medium (IMDM; GIBCO Invitrogen) with 10% heat-inactivated FBS and 8% conditioned WEHI-3b cell–conditioned medium as a source of IL-3.
Patient AML blast cell samples
Marrow samples were taken from adult patients in untreated first relapse of AML (non-M3 subtypes) who participated in the phase 2 clinical trials with GO.20,21 Thawed aliquots of frozen samples of density gradient-isolated mononuclear cells containing leukemic blast cells were used for these studies. All patients signed informed consents, and the institutional review boards of the participating institutions approved all protocols.
Lentiviral vectors and gene transfer
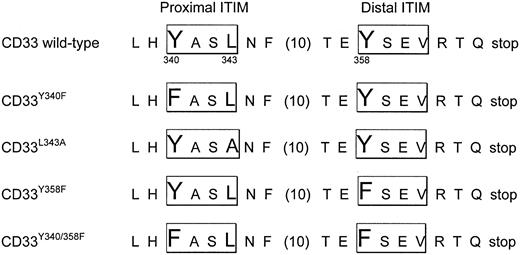
pcDNA3 vectors containing complementary DNA (cDNA) for wild-type CD33, CD33Y340F, CD33Y358F, and CD33Y340F/Y358F were kindly provided by Dr D. W. McVicar, National Cancer Institute, Frederick, MD.5 Site-directed mutagenesis with the primer 5′-ATG AGG AGC TGC ATT ATG CAT CCG CCA ACT TTC ATG GGA TGA ATC-3′ was performed to generate CD33L343A (Figure 6). These cDNAs were cloned into the pRRLsin.cPPT.MSCV vector as a CD33–internal ribosome entry site–enhanced green fluorescent protein (CD33-IRES-EGFP) cassette using standard polymerase chain reaction (PCR) cloning procedures. All constructs were verified by sequencing. The vector pRRLsin.cPPT.MSCV.EGFP was used as control in some experiments. Due to high levels of EGFP expression from this control vector, the maximal multiplicity of infection (MOI) used was 10. In a previous study, we found that human AML cell lines (ML-1, NB4, HL-60, U937) that were transduced with the control vector (used at an MOI up to 10) or the vector containing an irrelevant gene (used at an MOI up to 100) were similar to nontransduced cells with regard to cell viability and response to GO or the free calicheamicin-γ1 derivative.22 Stocks of vesicular stomatitis virus G glycoprotein (VSV-G)–pseudotype lentiviral vectors were prepared by calcium phosphate–mediated 3-plasmid transfection of 293T cells. Briefly, 27 μg of the transfer vector construct, 17.5 μg second generation gag-pol packaging construct pCMVR8.74, and 9.5 μg VSV-G expression construct pMD.G were used for transfection of 1.2 × 107 293T cells overnight in 25 mL Dulbecco modified Eagle medium (DMEM; GIBCO Invitrogen) with 10% heat-inactivated FBS. The cells were treated with 10 mM sodium butyrate during the first of 3 12-hour vector supernatant collections. The supernatant was filtered through a 0.22 μm pore-size filter and concentrated 100-fold by ultracentrifugation. All vector stocks were titered by transducing HT1080 cells using limiting dilutions of the stock with analysis for EGFP expression by flow cytometry. Transductions were performed in fibronectin-coated wells in the presence of 8 μg/mL protamine sulfate at an MOI of 1 to 100. EGFP-positive cells were sorted by flow cytometry and recultured for further analysis.
Schematic representation of CD33 mutants used in internalization and cytotoxicity experiments. Single-letter amino acid code is used. Spacing between F and T is shown in brackets.
Schematic representation of CD33 mutants used in internalization and cytotoxicity experiments. Single-letter amino acid code is used. Spacing between F and T is shown in brackets.
Flow cytometry assays for determination of CD33 expression and internalization
A cytofluorometric immunofluorescence staining method was used to characterize both the membrane display of CD33 of resting cells as well as the internalization of antibody-bound CD33. Cell surface CD33 expression in untreated cells was detected by staining with phycoerythrin (PE)–conjugated anti-CD33 antibody (clone P67.6; BD Biosciences, San Jose, CA). For a staining negative control, parallel samples were incubated with an irrelevant PE-conjugated isotype control antibody (BD Biosciences) at the same protein concentration. To measure internalization of antibody-bound CD33 in AML cell lines, cells (typically 1 × 106 to 1.5 × 106) were transferred into 5 mL polystyrene round-bottom tubes (BD Falcon, Discovery Labware, Bedford, MA) and incubated for at least 30 minutes with IMDM medium containing 10 μg/mL unconjugated, unlabeled hP67.6 (kindly provided by Wyeth-Ayerst Research, Radnor, PA) in ice water (to prevent internalization during the staining procedure). Cells were then washed in ice-cold phosphate-buffered saline (PBS; GIBCO Invitrogen), resuspended in IMDM medium without antibody, split into several tubes, and incubated at 37°C (in 5% CO2 and air) for various periods of time. Afterward, cells were chilled and incubated with biotin-conjugated mouse anti–human IgG4 monoclonal antibody (used at 5 μg/mL in PBS/2% FBS), followed by incubation with streptavidin-PE conjugate (used at 5 μg/mL in PBS/2% FBS; both from BD Pharmingen, San Diego, CA) to detect remaining hP67.6 on the cell surface. One sample that was kept in ice water was used to determine the starting level of antibody bound to the cell surface. The same assay was used to measure internalization of antibody-bound CD33 in primary AML blast cells; however, cells were kept in IMDM supplemented with 20% FBS and 100 ng/mL of the following recombinant human cytokines: granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor, IL-3, and granulocyte colony-stimulating factor (G-CSF) (all from Amgen, Thousand Oaks, CA) during the assay.21,23 To identify nonviable cells, all samples were stained with propidium iodide (PI; Sigma, St Louis, MO). At least 10 000 events were acquired and PI-negative cells were analyzed on a FACScan flow cytometer using Cellquest software (BD Biosciences). Linear fluorescence values were used to calculate the percentage of antibody internalization. Using this assay, a time-dependent loss of cell surface hP67.6 was observed in a panel of human CD33+ AML cell lines (ML-1, HL-60, U937, NB4, and TF-1 cells). To verify that the time-dependent loss of cell surface–associated hP67.6 reflects antibody internalization, as opposed to shedding or dissociation of hP67.6 from CD33, we repeated these experiments with hP67.6 that had been directly labeled with Alexa Fluor 488 (Molecular Probes, Eugene, OR). Alexa Fluor 488 is a green small molecular weight dye whose fluorescence properties are relatively independent of changes in surrounding pH. Cell-associated green fluorescence remained unchanged when the internalization assay was performed with Alexa Fluor 488–labeled hP67.6, while cell surface hP67.6 decreased during the assay, as detected by using biotin-conjugated mouse anti–human IgG4 monoclonal antibody and a streptavidin-PE conjugate. Thus, the loss of cell surface–associated hP67.6 measured over time in our assay indeed reflects antibody internalization.
Confocal microscopy
Cells were first incubated for 45 minutes in IMDM medium containing 20 μg/mL hP67.6 that was directly labeled with Alexa Fluor 594 (Molecular Probes) in ice water. Cells were then washed with ice-cold PBS, resuspended in IMDM medium without antibody, and incubated at 37°C for 30 minutes. Subsequently, cells were fixed with PermiFlow (Invirion, Frankfort, MI) at room temperature for 30 minutes, stained with DAPI (4,6 diamidino-2-phenylindole dihydrochloride), and mounted on polylysine-coated slides with SlowFade antifade solution (Molecular Probes). Cells were examined on a Deltavision SA3.1 wide-field deconvolution microscope system (Applied Precision, Issaquah, WA) using an Olympus 60 × 1.4 NA objective on an Olympus IX-70 inverted microscope (Olympus, Melville, NY), and images were acquired with a Photometrics CH350 cooled coupling device (CCD) camera (Photometrics, Tucson, AZ). Three-dimensional images were deconvolved using an iterative constrained algorithm (SoftWoRX software, Applied Precision).
Assays for drug-induced cytotoxicity
Cells were taken during exponential growth and incubated at 37°C (in 5% CO2 and air) in 96-well round-bottom plates (BD Falcon) at 2 × 104 cells per well in 240 μL culture medium containing various concentrations of GO or, in some experiments, N-acetyl-γ-calicheamicin dimethyl hydrazide (referred to as calicheamicin-γ1; both kindly provided by Wyeth-Ayerst Research). In some experiments with KG-1a cells, cyclosporine A (CSA; Sigma) was added at a concentration of 2.5 μg/mL. After 3 days of culture,18,23,24 cell numbers and drug-induced cytotoxicity, using PI to detect nonviable cells, were determined using a FACSCalibur flow cytometer (BD Biosciences) and analyzed with Cellquest software. In early studies, we used 2-hour exposures to GO, after which cells were washed and then resuspended in GO-free medium and incubated for 3 days before cytotoxicity was assessed, as previously published.23,24 In this assay, OCI-AML3 cells became more sensitive to GO when CD33 was overexpressed (in one experiment: +7% PI-positive cells in parental cells versus +30% PI-positive cells in cells transduced with an MOI of 50 at a dose of GO of 2.5 ng/mL). However, 32D cells remained resistant at the highest GO dose used (40 ng/mL for 2 hours) even when expressing high levels of CD33. KG-1a cells were totally resistant to a pulse of 40 ng/mL GO, even in the continuous presence of CSA (data not shown). We therefore exposed parental and transduced cells to continuous GO for 3 days to measure cytotoxicity. As a control for assaying the toxicity of GO, we first measured the toxicity of free calicheamicin-γ1. A direct comparison revealed that the sensitivity of the cell lines toward calicheamicin-γ1 varied greatly and was found to be in the following order: OCI-AML3 cells greater than 32D cells greater than KG-1a cells. For example, the fraction of PI-positive cells increased by 19.2% ± 5.5% at 0.25 ng/mL calicheamicin-γ1 and by 60.0% ± 13.1% at 2.5 ng/mL calicheamicin-γ1 in parental OCI-AML3 cells compared with cells incubated without cytotoxic drug (data given as mean ± SEM; n = 3), whereas the fraction of PI-positive 32D cells increased to a significant degree only at 5 ng/mL calicheamicin-γ1 (35.0% ± 11.3%). By comparison, KG-1a cells were completely resistant to 10 ng/mL calicheamicin-γ1 in the absence of CSA. Therefore, direct comparisons of cytotoxic effects of calicheamicin-γ1, and by extrapolation of GO, are not possible between different cell lines but need to be assessed in each cellular background individually.
Results
Effect of CD33 expression on GO-induced cytotoxicity
We first sought to determine the quantitative relationship between CD33 expression and the cytotoxic effect of GO in vitro. Although we have found significantly different levels of endogenous CD33 expression on a panel of human AML cell lines in a previous study,24 these cell lines also had different susceptibilities to the cytotoxicity induced by free calicheamicin-γ1 (see “Materials and methods”), rendering direct comparisons between individual cell lines with regard to GO-induced cytotoxicity impossible. Furthermore, an obvious limitation of comparisons between different cell lines is the fact that they may differ not only with regard to the level of CD33 expression but also with regard to other parameters that might be important for GO-induced cytotoxicity, such as drug internalization (see the next section), intracellular trafficking, or the presence of resistance mechanisms. To unequivocally assess the importance of CD33 expression, we therefore prepared sublines of a given cell type that were forced to express different levels of CD33 under the murine stem cell virus (MSCV) promoter via lentivirus-mediated gene transfer.
Although nearly all widely used human AML cell lines express endogenous CD33, a previous study found that the OCI-AML3 cell line lacks CD33.25 However, we found small but detectable levels of endogenous CD33 on parental OCI-AML3 cells, as determined by flow cytometry–based immunostaining (Figure 1). We also examined the weakly CD33-positive human myeloid KG-1a cell line and the murine 32D myeloid line, which is devoid of CD33.
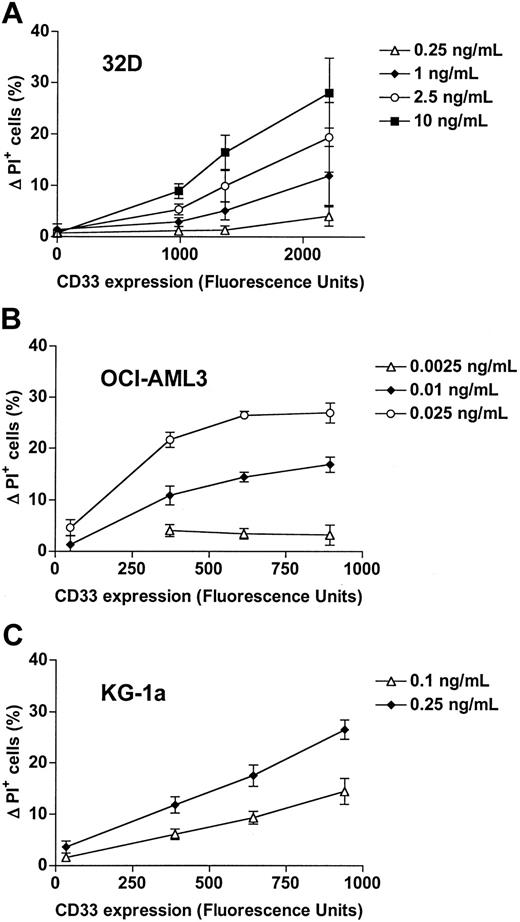
CD33 wild-type protein expression of parental and transduced cell lines. CD33 cell surface staining intensity, represented by the mean fluorescence intensity of cells stained with monoclonal anti-CD33 antibody (after subtraction of the mean fluorescence intensity of cells stained with isotype control antibody) of (A) murine 32D, (B) human OCI-AML3, and (C) human KG-1a cells. Results from one representative experiment are shown for each cell line. MOI denotes multiplicity of infection. Fluorescence intensity values cannot be directly compared between cell lines, because the brightness of the staining of CD33-transduced OCI-AML3 cells required data acquisition with modified voltage parameters.
CD33 wild-type protein expression of parental and transduced cell lines. CD33 cell surface staining intensity, represented by the mean fluorescence intensity of cells stained with monoclonal anti-CD33 antibody (after subtraction of the mean fluorescence intensity of cells stained with isotype control antibody) of (A) murine 32D, (B) human OCI-AML3, and (C) human KG-1a cells. Results from one representative experiment are shown for each cell line. MOI denotes multiplicity of infection. Fluorescence intensity values cannot be directly compared between cell lines, because the brightness of the staining of CD33-transduced OCI-AML3 cells required data acquisition with modified voltage parameters.
Lentivirus-mediated transfer of wild-type CD33 at an MOI of 1 to 100 in each of these cell lines yielded stable subpopulations of EGFP-positive cells, which were sorted by flow cytometry and expanded. Upon analysis, these cells showed cell surface expression of CD33 with an MOI-dependent increase in expression levels of CD33 (Figure 1). The range of fluorescence intensities in CD33-transduced cells was very narrow; that is, homogeneous populations of CD33-overexpressing cells could be generated (data not shown).
To assess the effect of CD33 expression on GO-induced cytotoxicity in each of the parental and CD33-transduced cell lines that express CD33 at different levels, cells were treated with various concentrations of GO for 3 days and cytotoxicity then determined by flow cytometry with PI. As shown in Figure 2, parental 32D cells, which do not express the CD33 antigen, are resistant to GO at concentrations that effectively kill CD33+ cells. By comparison, parental OCI-AML3 cells, which express low levels of endogenous CD33, are sensitive to GO at a dose-equivalent concentration (DEC) of about 0.01 to 0.025 ng/mL (Figure 2). Expression of the empty control vector did not significantly change the response to GO in these cells (data not shown). Parental KG-1a cells were completely resistant to GO (as well as the free toxic calicheamicin-γ1) at a concentration as high as 10 ng/mL DEC despite endogenous expression of low levels of CD33. Although resistance mechanisms to calicheamicin-γ1 were not investigated systematically in this study, KG-1a cells were found to express P-glycoprotein (Pgp or MDR1 or ABCB1), as detected by immunostaining with anti-Pgp antibody (clone 4E3.16)23 and a functional efflux assay with the fluorescent dye 3,3′-diethyloxacarbocyanine iodide (DiOC2) in the presence or absence of the Pgp inhibitor CSA23 (data not shown). Previous in vitro studies have demonstrated that Pgp-expressing cell lines and patient AML blast cells are resistant to GO and that Pgp inhibitors can restore drug sensitivity in Pgp-expressing cells.21,23,24,26,27 We therefore performed similar cytotoxicity assays with parental KG-1a cells in the presence of CSA to block Pgp-mediated drug efflux. With this cotreatment, these cells became susceptible to GO, as shown in Figure 2. In all 3 cell lines, however, lentivirus-mediated transfer of wild-type CD33 resulted in significantly enhanced sensitivity to GO. Importantly, GO sensitivity increased in an MOI-dependent manner—that is, in a direct correlation with the cell surface expression levels of CD33 (Figure 2); this MOI dependency was especially apparent at lower GO concentrations. Together, these data demonstrate the importance not only of CD33 expression per se but also of the level of CD33 expression for GO-induced cytotoxicity.
Effect of CD33 expression on GO-induced cytotoxicity. Parental (A) 32D, (B) OCI-AML3, and (C) KG-1a cells and their sublines that were transduced with wild-type CD33 and expressed CD33 at different levels, as indicated by arbitrary fluorescence units, were incubated with various concentrations of GO for 3 days, before cytotoxicity was assessed with PI staining. In the case of KG-1a sublines, cells were cotreated with CSA. Increases in the percentage of PI-positive cells in GO-treated cells are compared with corresponding cells that were incubated without GO, and results are shown as mean ± SEM from 2 to 4 independent experiments performed in duplicate or quadruplicate wells.
Effect of CD33 expression on GO-induced cytotoxicity. Parental (A) 32D, (B) OCI-AML3, and (C) KG-1a cells and their sublines that were transduced with wild-type CD33 and expressed CD33 at different levels, as indicated by arbitrary fluorescence units, were incubated with various concentrations of GO for 3 days, before cytotoxicity was assessed with PI staining. In the case of KG-1a sublines, cells were cotreated with CSA. Increases in the percentage of PI-positive cells in GO-treated cells are compared with corresponding cells that were incubated without GO, and results are shown as mean ± SEM from 2 to 4 independent experiments performed in duplicate or quadruplicate wells.
Measurement of internalization of antibody-bound CD33 in AML cell lines and primary AML blasts
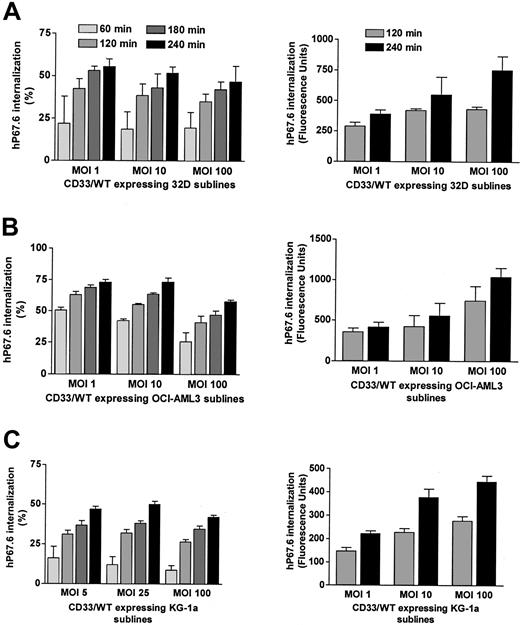
It has previously been established that antibody-bound CD33 is internalized by both CD33+ hematopoietic cell lines and primary AML blast cells.15-18 To quantify antibody uptake, we modified a flow cytometry–based assay18 in which cells labeled with unconjugated hP67.6 were allowed to internalize in antibody-free medium for various periods of time. Second- and third-step reagents were then used to measure hP67.6 that remained on the cell surface. Control experiments with directly labeled hP67.6 were performed to verify that the time-dependent loss of cell surface–associated hP67.6 is reflective of antibody internalization and not indicative of dissociation of the antibody from CD33 or shedding of the antigen (see “Materials and methods”). In addition, antibody internalization was visualized with confocal microscopy (data not shown and Figure 3). Using this flow cytometry–based assay, we could easily detect loss of cell surface–associated hP67.6—that is, internalization of antibody-bound CD33 in a panel of CD33+ human AML cell lines (data not shown) as well as in 32D, OCI-AML3, and KG-1a cells that were transduced with wild-type CD33. As shown in Figure 4, the loss of cell surface–associated hP67.6 ranged from about 10% to 15% to about 50% after 60 minutes of incubation at 37°C. The percentage of antibody-bound CD33 that was internalized was relatively independent of the levels of cell surface CD33 expression in 32D and KG-1a cells, whereas it was reduced in OCI-AML3 sublines that expressed high levels compared with sublines that expressed low levels of cell surface CD33 (Figure 4). This suggests that the endocytosis machinery is not saturated by overexpressed CD33, except perhaps in the case of OCI-AML3 sublines expressing high levels of CD33. Accordingly, the total amount of antibody internalization, as estimated by changes in arbitrary fluorescence units, was greater in sublines that expressed higher levels of CD33 (Figure 4, right panels).
Confocal microscopy study of ITIM-dependent internalization of antibody-bound CD33 in transduced cell lines. OCI-AML3 cell sublines transduced with either wild-type CD33 or CD33 mutants were incubated with Alexa Fluor 594–labeled hP67.6 on ice water before the cells were transferred to antibody-free medium and either kept in ice water (left panels) or incubated in 37°C (right panels) to allow internalization of antibody-bound CD33 for 30 minutes. Cells were then fixed, stained with DAPI, and visualized by confocal microscopy.
Confocal microscopy study of ITIM-dependent internalization of antibody-bound CD33 in transduced cell lines. OCI-AML3 cell sublines transduced with either wild-type CD33 or CD33 mutants were incubated with Alexa Fluor 594–labeled hP67.6 on ice water before the cells were transferred to antibody-free medium and either kept in ice water (left panels) or incubated in 37°C (right panels) to allow internalization of antibody-bound CD33 for 30 minutes. Cells were then fixed, stained with DAPI, and visualized by confocal microscopy.
Internalization of antibody-bound wild-type CD33 in transduced cell lines. (A) 3 2D cell sublines, (B) OCI-A ML3 cell sublines, and (C) KG-1a cell sublines transduced with wild-type CD33 at different MOIs were labeled with unconjugated hP67.6 on ice water before the cells were incubated in 37°C in antibody-free medium to allow internalization of antibody-bound CD33 up to 4 hours as indicated. Subsequently, remaining cell surface–associated hP67.6 was detected with biotin-conjugated mouse anti–human IgG4 monoclonal antibody and a streptavidin-PE conjugate. Each left panel shows the percentage of internalized hP67.6 relative to cells kept at 0°C, whereas the right panels depict total amount of internalized hP67.6, expressed as change in arbitrary fluorescence units. Results are shown as mean ± SEM from 2 to 5 independent experiments.
Internalization of antibody-bound wild-type CD33 in transduced cell lines. (A) 3 2D cell sublines, (B) OCI-A ML3 cell sublines, and (C) KG-1a cell sublines transduced with wild-type CD33 at different MOIs were labeled with unconjugated hP67.6 on ice water before the cells were incubated in 37°C in antibody-free medium to allow internalization of antibody-bound CD33 up to 4 hours as indicated. Subsequently, remaining cell surface–associated hP67.6 was detected with biotin-conjugated mouse anti–human IgG4 monoclonal antibody and a streptavidin-PE conjugate. Each left panel shows the percentage of internalized hP67.6 relative to cells kept at 0°C, whereas the right panels depict total amount of internalized hP67.6, expressed as change in arbitrary fluorescence units. Results are shown as mean ± SEM from 2 to 5 independent experiments.
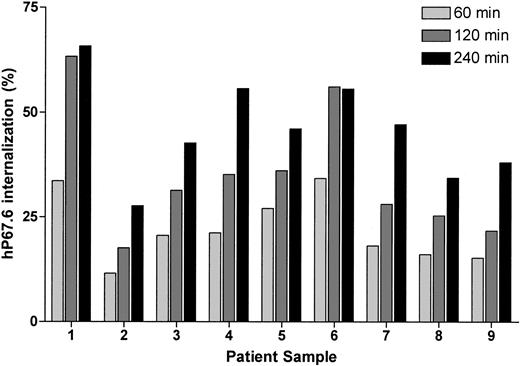
We also measured internalization of antibody-bound CD33 in 9 AML blast cell samples. As shown in Figure 5, the loss of cell surface–associated hP67.6 varied about 3-fold between between individual samples; thus, these values were comparable to those determined in AML cell lines expressing endogenous CD33 and AML cell lines transduced with wild-type CD33.
Internalization of antibody-bound wild-type CD33 in primary AML blast cells. Primary AML blast cells were labeled with unconjugated hP67.6 on ice water before the cells were incubated at 37°C in antibody-free medium to allow internalization of antibody-bound CD33 up to 4 hours as indicated. Subsequently, remaining cell surface–associated hP67.6 was detected with biotin-conjugated mouse anti–human IgG4 monoclonal antibody and a streptavidin-PE conjugate. Shown is the percentage of internalized hP67.6 relative to cells kept at 0°C.
Internalization of antibody-bound wild-type CD33 in primary AML blast cells. Primary AML blast cells were labeled with unconjugated hP67.6 on ice water before the cells were incubated at 37°C in antibody-free medium to allow internalization of antibody-bound CD33 up to 4 hours as indicated. Subsequently, remaining cell surface–associated hP67.6 was detected with biotin-conjugated mouse anti–human IgG4 monoclonal antibody and a streptavidin-PE conjugate. Shown is the percentage of internalized hP67.6 relative to cells kept at 0°C.
ITIM-dependent internalization of antibody-bound CD33 in transduced cell lines
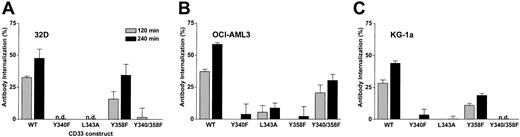
We next sought to determine the motif or motifs that control internalization of antibody-bound CD33. For CD22, a B-cell–restricted member of the siglec family, it has recently been shown that antibody-mediated internalization of the antigen is dependent on the integrity of 2 ITIM motifs.28 Given the similarity to CD33, we hypothesized that the ITIM motifs on the cytoplasmic tail of CD33 may control antibody-bound internalization of CD33. To test this hypothesis, we expressed a series of CD33 mutants in 32D, OCI-AML3, and KG-1a cells: CD33Y340F, in which the tyrosine of the first ITIM motif at position 340 was changed to phenylalanine; CD33L343A, in which the leucine contained in the proximal ITIM motif at position 343 was changed to alanine; CD33Y358F, in which the tyrosine of the distal ITIM motif at position 358 was changed to phenylalanine; and CD33Y340F/Y358F, in which the tyrosines of both ITIM motifs were changed to phenylalanine (Figure 6). Lentivirus-mediated transfer of these CD33 mutants at an MOI of 10 to 50 yielded stable subpopulations of EGFP-positive cells, which, after sorting and expansion, showed comparable levels of cell surface expression of CD33 (not shown). As shown in Figure 7, disruption of the proximal ITIM motif, either by changing tyrosine to phenylalanine (CD33Y340F mutant) or by changing leucine to alanine (CD33L343A mutant), resulted in an almost complete abrogation of internalization of antibody-bound CD33 in transduced OCI-AML3, KG-1a, and 32D cells. Slightly divergent results were found when the distal ITIM motif was disrupted: in 32D and KG-1a cells, internalization was only partially inhibited, whereas in OCI-AML3 cells, internalization was completely disrupted in cells expressing CD33Y358F. Similar to sublines expressing a mutant proximal ITIM, 32D and KG-1a cells expressing mutated proximal and distal ITIMs (CD33Y340F/Y358F mutant) completely failed to internalize antibody-bound CD33. In contrast, in the OCI-AML3 subline expressing CD33Y340F/Y358F, internalization of antibody-bound CD33 was surprisingly only partially inhibited. Internalization of antibody-bound CD33 was also studied visually by use of confocal microscopy, and similar results were obtained, as shown in Figure 3 for OCI-AML3 cells. Together, these data indicate that the proximal ITIM motif and, at least to some degree, also the distal ITIM motif control internalization of antibody-bound CD33 in myeloid cell lines.
ITIM-dependent internalization of antibody-bound CD33 in transduced cell lines. (A) 32D sublines, (B) OCI-AML3 sublines, and (C) KG-1a sublines, transduced with either wild-type CD33 or CD33 mutants, were labeled with unconjugated hP67.6 on ice water before the cells were incubated at 37°C in antibody-free medium to allow internalization of antibody-bound CD33 for 2 (shaded bars) or 4 hours (filled bars). Subsequently, remaining cell surface–associated hP67.6 was detected with biotin-conjugated mouse anti–human IgG4 monoclonal antibody and a streptavidin-PE conjugate. Results are shown as mean ± SEM from 3 to 4 (for 32D and KG-1a sublines) or 2 (for OCI-AML3 sublines) independent experiments. n.d. indicates no internalization detected.
ITIM-dependent internalization of antibody-bound CD33 in transduced cell lines. (A) 32D sublines, (B) OCI-AML3 sublines, and (C) KG-1a sublines, transduced with either wild-type CD33 or CD33 mutants, were labeled with unconjugated hP67.6 on ice water before the cells were incubated at 37°C in antibody-free medium to allow internalization of antibody-bound CD33 for 2 (shaded bars) or 4 hours (filled bars). Subsequently, remaining cell surface–associated hP67.6 was detected with biotin-conjugated mouse anti–human IgG4 monoclonal antibody and a streptavidin-PE conjugate. Results are shown as mean ± SEM from 3 to 4 (for 32D and KG-1a sublines) or 2 (for OCI-AML3 sublines) independent experiments. n.d. indicates no internalization detected.
Correlation between internalization of CD33 and GO-induced cytotoxicity
Finally, we investigated whether internalization of CD33 correlates with the extent of GO-induced cytotoxicity. For these experiments, we treated 32D, OCI-AML3, and KG-1a cells that were transduced with either wild-type CD33 or CD33 mutants with GO for 3 days, before cytotoxicity was assessed with PI staining. Because our initial experiments have indicated the importance of the expression levels of CD33 for GO-induced cytotoxicity, we used sublines expressing comparable levels of CD33 for these assays.
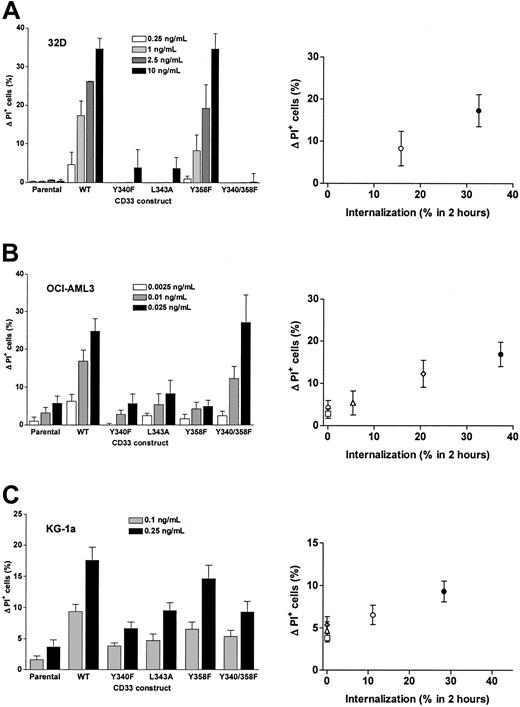
As shown in Figure 8, disruption of the proximal ITIM-like motif or both motifs resulted in an almost complete abrogation of GO-induced cytotoxicity in 32D cells, whereas disruption of the distal ITIM-like motif only partially abrogated GO-induced cytotoxicity, thus very well correlating to the internalization properties of the CD33 mutants (compare Figure 8A with Figure 7A). OCI-AML3 cells transduced with CD33 mutants with a disrupted proximal or distal ITIM-like motif were about as sensitive to GO-induced cytotoxicity as parental cells, whereas OCI-AML3 cells transduced with wild-type CD33 (or the CD33Y340-358F mutant) were clearly more sensitive to GO; again, sensitivity to GO very well correlated with the internalization properties of the CD33 mutants (compare Figure 8B with Figure 7B). Similar results were obtained for the KG-1a sublines (in which cells were cotreated with CSA), although the effect of disruption of the ITIM-like motifs was less pronounced than in the 32D or OCI-AML3 sublines (compare Figure 8C with Figure 7C). Together, these data indicate that disruption of the proximal ITIM-like motif and, at least to some degree, also the distal ITIM-like motif significantly decrease GO-induced cytotoxicity compared with cells expressing wild-type CD33 at comparable levels.
ITIM-dependent GO-induced cytotoxicity in transduced cell lines. (A) 32D cell sublines, (B) OCI-AML3 cell sublines, and (C) KG-1a cell sublines transduced with either wild-type CD33 or CD33 mutants were incubated with indicated concentrations of GO for 3 days, before cytotoxicity was assessed with PI staining. In the case of KG-1a sublines, cells were cotreated with CSA. Increases in the percentage of PI-positive cells in GO-treated cells are compared with corresponding cells that were incubated without GO, and results are shown as mean ± SEM from 2 to 5 independent experiments performed in duplicate or quadruplicate wells. The left panels show the data grouped for individual sublines, whereas the right panels show the effect of GO-induced cytotoxicity in relation to the percentage of internalization of CD33 after 2 hours.
ITIM-dependent GO-induced cytotoxicity in transduced cell lines. (A) 32D cell sublines, (B) OCI-AML3 cell sublines, and (C) KG-1a cell sublines transduced with either wild-type CD33 or CD33 mutants were incubated with indicated concentrations of GO for 3 days, before cytotoxicity was assessed with PI staining. In the case of KG-1a sublines, cells were cotreated with CSA. Increases in the percentage of PI-positive cells in GO-treated cells are compared with corresponding cells that were incubated without GO, and results are shown as mean ± SEM from 2 to 5 independent experiments performed in duplicate or quadruplicate wells. The left panels show the data grouped for individual sublines, whereas the right panels show the effect of GO-induced cytotoxicity in relation to the percentage of internalization of CD33 after 2 hours.
Discussion
The data presented in this report support 3 major conclusions. First, we demonstrate the importance not only of CD33 expression per se but also of the level of CD33 expression for GO-induced cytotoxicity. Second, we identify the ITIMs as the amino acid sequences that largely control internalization of antibody-bound CD33. Finally, we found that disruption of these motifs by introduction of point mutations not only significantly reduced internalization of anti-CD33 antibody but also significantly reduced GO-induced cytotoxicity. Together, these data show an important role of both the number of CD33 molecules expressed on the cell surface and the amount of internalization of antibody for entry of the immunoconjugate into the cell and GO-induced cytotoxicity.
Previous in vitro studies have demonstrated a selective killing of CD33+ AML cells compared with CD33- lymphoblastic cells with GO.26,29 In line with these findings, we show in the present study that lentivirus-mediated expression of CD33 in 32D cells renders these cells susceptible to GO-induced cytotoxicity in vitro at concentrations that can be achieved after application of GO in patients in vivo.30 Such a comparison of parental CD33- cells with transduced CD33+ cells offers a major advantage over previous comparisons between different cell types, because it minimizes the chance of misinterpreting results due to confounding variables, such as cellular differences with regard to behavior of antigen after antibody is bound, trafficking, or drug resistance mechanisms. In addition to demonstrating the importance of CD33 per se, our data show that GO sensitivity directly correlated with the cell surface expression levels of wild-type CD33 in transduced OCI-AML3, KG-1a, and 32D cells. These data clearly indicate a role of the cell surface expression level of CD33 as a limiting factor for the cytotoxic effect of GO in CD33-transduced myeloid cell lines. Such an effect may be difficult to detect in primary AML blast cells for above-mentioned reasons, although quantitative flow cytometry studies have shown that the level of CD33 expression varies widely between individual patients with AML.31 In fact, a correlation between marrow blast CD33 antigen levels and clinical response was not observed in clinical trials using GO monotherapy for CD33+ relapsed adult AML.20,32 In these phase 2 trials, however, only patients with predefined levels of CD33 (ie, more than 80% of leukemic blast cells with CD33 immunofluorescence staining 4 times above background staining was required20 ) were included; in addition, the analysis may be confounded by other factors, such as multidrug resistance, particularly Pgp expression. Together with these findings, our data suggest that manipulation of the expression level of CD33 on AML blasts may be a first novel therapeutic approach to improve clinical efficacy of GO. Unfortunately, the regulation of CD33 expression on both normal and malignant cells is not understood at present. Preliminary in vitro studies suggested that G-CSF, GM-CSF, interferon-α, IL-3, and IL-6 may modestly increase CD33 expression on the surface of CD34+/CD33+ blasts,33 but additional more detailed experiments are needed to fully explore ways to manipulate CD33 expression on AML blasts. Furthermore, previous studies have indicated that reexpression of CD33, following internalization of antibody-bound CD33, significantly contributes to the total amount of internalized anti-CD33 antibody.18 It seems likely that the rate of reexpression of CD33 may be regulated. If so, variations in the rate of reexpression would impact the total amount of antibody internalization and, thus, GO toxicity.
Recent studies have shown that drug efflux mediated by members of the adenosine triphosphate (ATP) binding cassette (ABC) superfamily of proteins, predominantly Pgp but, to a lesser degree, also multidrug resistance protein (MRP), mediate resistance to GO.20,21,23,24,26,27 In line with the perceived importance of Pgp for resistance to GO, the Pgp-positive KG-1a cells were completely resistant to GO at clinically useful doses. More importantly, although enforced overexpression of CD33 sensitized KG-1a cells in direct correlation to the level of CD33 expression, this effect on GO-induced cytotoxicity was only readily visible upon cotreatment with CSA, most likely because the capacity of drug efflux exceeded the amount of drug internalization. These findings emphasize the fact that increased accumulation of intracellular calicheamicin-γ1 can be achieved by either increased uptake of GO (eg, via increased expression of cell surface CD33) or decreased efflux of calicheamicin-γ1 (eg, via inhibition of ABC transporters) or both.
In line with previous studies, anti-CD33 antibody was internalized after engagement by cells expressing wild-type CD33.15-18 Antibody internalization was relatively independent of the expression level of CD33 in 32D and KG-1a cells. By comparison, the percentage of antibody internalization dropped in OCI-AML3 sublines that expressed high levels of CD33. This is not necessarily unexpected, because it has been repeatedly observed that the endocytic machinery can be saturated due to the limited availability of recognition components.34
Our experiments with CD33 mutants indicate that the proximal ITIM-like motif and, at least to some degree, also the distal ITIM-like motif control internalization of antibody-bound CD33 in transduced myeloid cell lines. Recent studies have indicated that these motifs are able to bind and activate SHP-1 and SHP-2 and to transmit inhibitory signals, at least under artificial in vitro conditions, if the tyrosines become phosphorylated.3-5 Additional experiments are needed to clarify whether these signaling events can occur in vivo (eg, in patients after treatment with GO) and to test whether outside-in signaling is linked to or is independent from receptor internalization.
The mechanism(s) underlying internalization of antibody-bound CD33 remains elusive. Mammalian cells have evolved several mechanisms for uptake of extracellular material and membrane components. However, endocytosis via clathrin-coated pits is by far the predominant mechanism for internalization of cell surface receptors and their cargo.35 Indeed, the ITIM-like motifs of CD33 resemble YXXΦ motifs (where X stands for any amino acid and Φ stands for an amino acid residue with a bulky hydrophobic side chain), a major tyrosine-based signal involved in clathrin-mediated protein sorting. This signal has been shown to interact with the μ subunit of the adaptor protein (AP) complex AP-2 and mediate clathrin-dependent endocytosis of, for example, the transferrin receptor and the asialoglycoprotein receptor.34 Similarly, it has recently been shown that antibody-mediated internalization of the B-cell coreceptor CD22, another ITIM-bearing siglec, is dependent on the integrity of 2 ITIMs and is mediated by AP-2.28 The Y residue is critical as anchor for the interaction with AP-2, whereas the Φ position can accommodate several residues with bulky hydrophobic side chains.34 Our finding that both the CD33Y340F mutant and the CD33L343A mutant did not internalize efficiently when bound with antibody would be consistent with an important role of those 2 residues and would fit the recognition pattern of the YXXΦ motif with the μ subunit of AP-2, and it is therefore tempting to speculate that AP-2 may be involved in internalization of antibody-bound CD33. However, alternate endocytic adaptors have been described whose interaction depends on the presence of (phospho-) tyrosine motifs,36,37 and additional studies will be required to dissect out the mechanisms that are involved in internalization of antibody-bound CD33 and to elucidate the role of tyrosine phosphorylation for effective internalization. The finding that the CD33Y340/358F mutant did internalize when expressed in OCI-AML3 cells but not in KG-1a or 32D cells cannot be explained satisfactorily at this time. We can speculate, however, that the disrupted tertiary structure of this protein, or a heterodimer formation with endogenous wild-type CD33, enabled the efficient binding of an adaptor protein of the endocytic machinery and may suggest differences in the posttranslational processing of CD33 or differences in the endocytic machinery in OCI-AML3 cells.
Our findings are limited because primary AML cells were not used. Preliminary studies on internalization of antibody-bound CD33 in 9 primary blast samples from patients with AML showed that between about 25% and 75% of antibody-bound CD33 was internalized over the first 4 hours, a range that is very comparable to what we found in our AML cell lines (data not shown). Although the levels of cell surface CD33 expression were quite different in these samples, meaningful comparisons of GO cytotoxicity between individual AML blast cell samples were not possible for obvious reasons (eg, differences in expression patterns of drug resistance proteins). The limited number of primary blast cells that were available per patient sample precluded studying endocytosis and GO toxicity effects of expressing additional wild-type or mutant CD33. We are hoping, however, that once the mechanism underlying internalization of antibody-bound CD33 has been elucidated in engineered AML cell lines, primary AML cells can be used to study whether such mechanisms are also operative in primary cells.
An important conclusion of this study is that not only the level of CD33 expression but also the rate of endocytosis determines the extent of GO-induced cytotoxicity. Manipulation of the endocytic process therefore appears as a second interesting pharmacologic target, because a faster internalization of antibody-bound CD33 may theoretically lead to enhanced GO-induced cytotoxicity, which in turn could lead to improved clinical outcomes of patients treated with GO.
Prepublished online as Blood First Edition Paper, September 28, 2004; DOI 10.1182/blood-2004-07-2784.
Supported in part by research funding from the Leukemia and Lymphoma Society (7040-03), the National Cancer Institute (CA92316), and the National Institutes of Health (DK56465 and GM066257). R.B.W. is the recipient of an Interdisciplinary Dual Mentor Fellowship from the Fred Hutchinson Cancer Research Center. I.D.B. is the recipient of an FM Kirby/American Cancer Society Clinical Research Professorship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Steven J. Collins and Daniel W. McVicar for helpful discussions as well as Hans-Peter Kiem and Jesse Thompson (Gene Marking and Tracking Facility, Core Center of Excellence in Molecular Hematology, Fred Hutchinson Cancer Research Center, Seattle, WA) for preparation of lentiviral vector stocks.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal