Comment on Kanazawa et al, page 1195
Missense mutations of Nod2/CARD15 are associated with the development of Blau syndrome. Kanazawa and colleagues provide evidence that similar mutations of Nod2 are associated with early-onset sarcoidosis, demonstrating a casual link between these 2 diseases.
In response to pathogen exposure, vertebrates have evolved multiple immune defense systems to eliminate the invasive microbe. However, genetic mutations in the molecules involved in these responses can lead to inappropriate immune responses and result in inflammatory disease. In this issue of Blood, Kanazawa and colleagues demonstrate a genetic link between missense mutations of one such immune defense molecule, Nod2, and the inflammatory disease early-onset sarcoidosis (EOS).
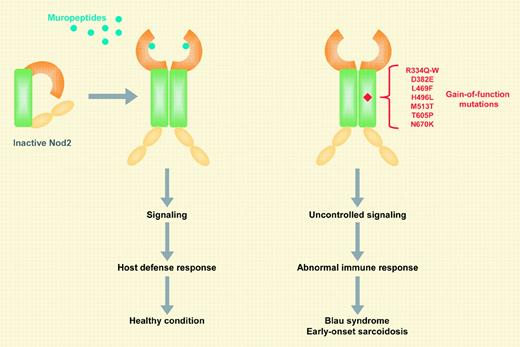
The sensing of microbial agents by the host is mediated through pathogen-recognition molecules (PRMs) that are activated by highly conserved structures uniquely found in microbes. A major class of PRMs is the Toll-like receptors (TLRs), which upon activation induce intracellular signaling pathways leading to microbicidal host responses. Another more recently identified class of PRMs is the nucleotide-binding oligomerization domain (NOD)–containing proteins (Nods). Unlike TLRs, Nods are primarily located inside the cell and sense microbial products in the cytosol.FIG1
Mutations in the coding region of a Nod family member, Nod2/Card15, have been implicated in 2 diseases characterized by granulomatous inflammation. Three major Nod2 mutations, R702W, G908R, and L1007fsinsC, as well as multiple rare variants, have been found to be associated with susceptibility to Crohn disease (CD), a chronic inflammatory bowel disease.1 These CD-associated Nod2 variants are deficient in their ability to sense muropeptides, the bacterial molecules recognized by Nod2.1,2 Upon stimulation, Nod2 activates signaling pathways, including nuclear factor–κB (NF-κB; see figure). In addition to CD, several missense mutations of Nod2 have been associated with the development of Blau syndrome (BS), a dominantly inherited disease characterized by early-onset arthritis, uveitis, and skin rashes.3
EOS is another rare but sporadic disease and shares several clinical and pathologic features with BS. This similarity suggests that both syndromes might represent hereditary and sporadic forms of the same disease. However, genetic evidence linking BS and EOS was lacking until now. The paper by Kanazawa and colleagues demonstrates that missense mutations of Nod2 are associated with the development of EOS, including the R334W mutation that was previously found in patients with BS.3 As previously reported for BS-associated proteins,2,4 all of the EOS-associated gene variants act as constitutively active Nod2 mutants. The disease-causing Nod2 mutations involved residues located in the NOD domain. The NOD domain is critically involved in oligomerization and activation of Nod2, suggesting that these mutations may stabilize the active conformation of Nod2. Notably, 4 of the 5 EOS-causing Nod2 mutations (R334W, D382E, M513T, and N670C) correspond to the mutated residues R260Q, D303N, A439V, and Y570C in cryopyrin, another Nod protein whose missense mutations are associated with development of inherited autoinflammatory disorders.1 There appears to be minor genetic heterogeneity within EOS, as no amino acid alterations of Nod2 have been found in 1 of the 10 reported Japanese patients and in 2 European patients originally reported by Miceli-Richard et al.3 Better understanding of the Nod2 signaling pathway might lead to more rational and successful treatments of Nod2-associated diseases. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal