Abstract
The chemokines platelet factor 4 (PF4) and RANTES (regulated on activation normal T cell expressed and secreted) are secreted by activated platelets and influence multiple cell types and biologic processes. For instance, PF4 inhibits progenitor cell proliferation and angiogenesis, while platelet-derived RANTES is involved in vascular recruitment of monocytes. However, little is known about functional interactions of PF4 and RANTES. Here we show that the presence of PF4 enhanced the arrest of RANTES-stimulated monocytes and monocytic cells on activated endothelial cells under flow conditions, while binding of PF4 to the monocyte surface was increased by RANTES. Both RANTES-triggered arrest and PF4 binding involved monocytic chondroitin sulfate. Ligand blots and surface plasmon resonance revealed a robust heterophilic interaction of PF4 with RANTES but not with RANTES variants defective in higher order oligomerization. The tetrameric mutant E26A bound to the monocyte surface without increasing PF4 binding, and monocyte arrest induced by E26A-RANTES was not enhanced by PF4. Stimulation of monocytes with supernatants of activated platelets triggered arrest involving RANTES and PF4, as shown by inhibition studies. Our results suggest that heterophilic interactions with PF4 require structural motifs important in RANTES oligomerization and amplify RANTES-triggered effects on monocyte adhesion. This may have implications for the modulation of inflammatory recruitment by platelet-derived chemokines.
Introduction
The chemokines RANTES (regulated on activation normal T cell expressed and secreted; CC-chemokine ligand 5 [CCL5]) and platelet factor 4 (PF4; CXC-chemokine ligand 4 [CXCL4]) are both released from the α-granules of activated platelets.1 Chemokines are known to govern multiple biologic processes and are classified into CXC-chemokines (eg, PF4) and CC-chemokines (eg, RANTES) according to the position of N-terminal cysteine residues. PF4 exerts chemotactic activities on fibroblasts, neutrophils, and monocytes2,3 ; inhibits angiogenesis and proliferation of hematopoietic progenitor cells4-6 ; promotes adhesion of neutrophils on endothelial cells3 ; and supports adhesion of hematopoietic progenitor cells under static conditions.7 Since the latter effect was not observed after exposure to chondroitinase, it may not be mediated by a classical high affinity 7-transmembrane domain chemokine receptor.7 While a specific heptahelical receptor with high affinity for PF4 has been elusive, some functions of PF4 have recently been explained by binding to an alternatively spliced form of the receptor CXCR3, which has been found to be expressed on endothelial cells (ECs).8
The capability to induce adhesion and transmigration of monocytes and T cells is a well-established feature of RANTES.9-11 For instance, both diffusible and anchored forms of RANTES have been found to regulate T-cell adhesion to subendothelial extracellular matrix.12 Moreover, recombinant or platelet-derived RANTES can bind to the surface of activated endothelium, where it triggers the firm arrest and transmigration of monocytes under flow conditions11,13 by engaging its receptors CC-chemokine receptor 1 (CCR1) and CCR5, respectively.14-16 These properties of RANTES may be instrumental for its involvement in atherogenic processes, such as neointima formation after arterial injury.17 In addition, both RANTES and PF4 deposited by circulating platelets have been implicated in the development of atherosclerotic lesions in apolipoprotein E–deficient mice.18
Like most chemokines, RANTES and PF4 bind glycosaminoglycans (GAGs) such as heparin, chondroitin sulfate, and dermatan sulfate.19,20 Mutations of the BBXB motif in RANTES result in profoundly impaired binding to heparin and decreased CCR1 activating capacity, thus identifying a principal site for GAG binding and control of receptor selectivity.20,21 In addition, alterations in this motif have been shown to significantly attenuate RANTES aggregation.21 The propensity to form large aggregates (> 200 kDa) is an essential feature of RANTES contributing to its recruitment function in vivo.22 It is noteworthy in this respect that the tetrameric RANTES mutant E26A retains in vivo activity, indicating a minimal structural requirement for proper function.22
PF4 has been found to interact with other heparin-binding molecules such as basic fibroblast growth factor-2 (bFGF2), vascular endothelial growth factor (VEGF), antithrombin, protein C, and interleukin-8 (IL-8).7,23-27 The interaction of PF4 and IL-8 has been well characterized and is likely to account for the inhibition of IL-8 signaling by PF4 in hematopoietic progenitor cells.7 However, the precise mechanism and functional consequences of this effect have not been fully elucidated.
Unlike IL-8, RANTES and PF4 are both abundantly stored in α-granules and secreted upon stimulation of platelets and thus have ample possibilities for interaction. Hence, we were prompted to study the combined effects of PF4 and RANTES in modulating adhesion of monocytes and the underlying mechanisms.
Materials and methods
Cells and reagents
Human umbilical vein endothelial cells (HUVECs) and growth media were purchased from PromoCell (Heidelberg, Germany) and monocytic Mono Mac 6 (MM6) cells were cultured as described.28 Monocytes were isolated from buffy coats of healthy donors by hyperosmotic NycoPrep 1.068 (Axis-Shield, Oslo, Norway) density gradient centrifugation.28 Platelets were isolated from healthy young donors without medication by differential centrifugation to a final concentration to 1012/L. Plasma was removed by washing platelets twice in Krebs Ringer buffer (4 mM KCl, 100 mM NaCl, 20 mM NaHCO3, 2 mM Na2SO4, 4.7 mM citric acid, 14.2 mM sodium citrate, pH 5), and platelets were activated with human thrombin (1 U/mL) in Hanks balanced salt solution, 10 mM Hepes (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 0.5% bovine serum albumin (BSA), pH 7.4 (HH), for 10 minutes at 37°C. After centrifugation, supernatants were sterile-filtered and incubated with MM6 cells for 5 minutes at 37°C. Human RANTES and mutants were prepared as described20,22 or were purchased from Peprotech (Rocky Hill, NJ). Platelet-purified human PF4 was obtained from ChromaTec (Greifswald, Germany). Unless stated, reagents were from Sigma-Aldrich (St Louis, MO).
Laminar flow assay
Laminar flow assays were performed as described.29 Confluent HUVECs activated with IL-1β (10 ng/mL for 12 hours) were assembled as the lower wall of a flow chamber on the stage of an Olympus IX 50 microscope (Olympus Optical, Hamburg, Germany). MM6 cells or monocytes (106 cells/mL) were resuspended and pretreated for 30 minutes with Met-RANTES (1 mg/mL in HH), neutralizing rabbit polyclonal anti-PF4 antibody (10 μg/mL in HH; Chemicon, Temecula, CA), or HH alone, and stimulated with RANTES, PF4 (200 ng/mL), both chemokines, or supernatants of activated platelets. Some MM6 cells were pretreated with chondroitinase ABC (0.5 U/mL), heparinase I, or heparinase III (1 U/mL) for one hour at 37°C. Prior to assays, CaCl2 and MgCl2 were added to a final concentration of 1 mM, and cells were perfused at 1.5 dyne/cm2. The number of monocytes firmly adherent by primary interaction with ECs after 4 minutes of accumulation was quantified in multiple fields by analysis of images recorded with a JVC 3CCD video camera and recorder (JVC, Wayne, NJ).
Flow cytometry
MM6 cells were incubated with chemokines (1 μg/mL) in HH for 45 minutes or with supernatant of activated platelets overnight. Some cells were pretreated with chondroitinase ABC (0.5 U/mL) for one hour at 37°C. Cells were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 15 minutes. Nonspecific binding was blocked by incubation with 1% BSA. Monoclonal antibody VL1 (Caltag, Burlingame, CA) and anti-PF4 were applied for 45 minutes at 1 μg/mL in PBS with 0.1% BSA. Secondary fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies (Sigma-Aldrich) were used for detection and cells were analyzed using a FACScalibur cytometer (Becton Dickinson cytometry, Palo Alto, CA).
Ligand blot
Of each chemokine, 3 μg was applied onto a Hybond nitrocellulose membrane (Amersham, Freiburg, Germany) and dried at room temperature. After blocking the membrane with 5% nonfat milk in Tris-buffered saline (TBS) (150 mM NaCl, 25 mM Tris [tris(hydroxymethyl)aminomethane]–Cl, pH 7.5) for 30 minutes, PF4 (1 μg/mL) binding reactions were performed in the same buffer with 0.05% Tween20 added. After extensive washing with TBS, membranes were incubated with primary anti-PF4 antibody (0.5 μg/mL) and subsequently with secondary goat antirabbit antibody coupled to horseradish peroxidase (1:20 000). The reactivity was evaluated using supersignal solution (Pierce, Rockford, IL) and visualized with scientific imaging film (XAR5, Kodak, Rochester, NY).
Surface plasmon resonance analysis
RANTES showed high nonspecific binding to the carboxymethylated dextran matrix of commonly used CM5 sensorchips. Therefore, a C1 sensorchip without dextran (Biacore AB, Uppsala, Sweden) was treated essentially as described.21 There were 2 flowcells of a C1 chip activated by injecting 50 μL ethyl(dimethylaminopropyl)carbodiimide/N-hydroxy-succinimide (0.2 M/0.05 M; Pierce), and 20 μL streptavidin (0.2 mg/mL) was subsequently perfused over the activated surface. The surface was subsequently inactivated by 4 successive injections of 20 μL ethylene diamine (1 M, pH 12). Human PF4 biotinylated at the N-terminus (bPF4) was chemically synthesized using solid-phase peptide synthesis and native chemical ligation as described.30 A detailed outline will be published elsewhere (W.A., P.v.H, R.R.K., C.W., T.M.H., manuscript in preparation, 2004). Synthetic bPF4 was injected over the first flowcell and 240 resonance units (RUs) were captured. The second flowcell was left untreated with bPF4 and used as a reference cell. Binding of RANTES or its mutants to bPF4 was assessed by injecting increasing concentrations and monitoring binding for up to 2 hours to obtain binding approximating steady state. The coupling procedure and measurements were performed in a Biacore 2000 device (Biacore AB) at a flow rate of 5 μL/min. Sensorgrams of RANTES binding were corrected for nonspecific background signal using BIAevaluation 3.0 software (Biacore AB) and equilibrium RUs were determined for each injection.
Statistical analysis
Data were presented as mean ± SEM of at least 3 independent experiments, and analyzed using GraphPad Prism (GraphPad software, San Diego, CA). Statistical analysis was performed by one-way analysis of variance (ANOVA) with Newman-Keuls correction for multiple comparison. Differences with P values less than .05 were considered statistically significant.
Results
Arrest of MM6 after stimulation with chemokines
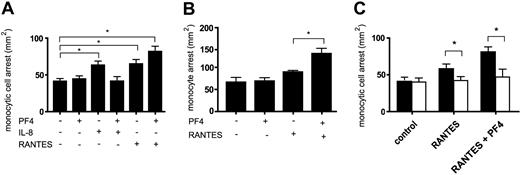
Monocyte arrest on endothelium can be triggered by chemokines including growth-related oncogene α (GRO-α), IL-8, and RANTES.11,28,31 To determine the functional consequences of a recently described interaction of IL-8 with PF4,7 we performed adhesion assays on activated HUVECs in a parallel wall flow chamber under flow conditions. Stimulation of monocytic MM6 cells with PF4 alone did not increase their arrest, whereas stimulation with IL-8 increased firm monocyte arrest by more than 50% (Figure 1A). In line with the abrogation of IL-8 signaling by PF4,7 the addition of PF4 completely suppressed IL-8–triggered monocyte arrest (Figure 1A), suggesting that an interaction of both chemokines may prevent IL-8 from activating its receptor. To test whether the inhibition by PF4 is specific for IL-8 or extends to other chemokines, we used RANTES to induce monocyte arrest. Stimulation of MM6 cells with RANTES alone significantly increased their arrest. However, the combination with PF4 resulted in a 2-fold enhancement of RANTES-triggered monocyte arrest (Figure 1A). The amplification of RANTES-triggered arrest was even more pronounced in isolated human blood monocytes, where at submaximal concentrations only the combination of RANTES with PF4 significantly increased arrest on activated HUVECs (Figure 1B).
Influence of PF4 on the arrest of chemokine-activated monocytes under flow conditions. Monocytic MM6 cells (A) or isolated human blood monocytes (B) were left untreated (control) or stimulated with indicated chemokines (200 ng/mL) (A-B). The relevance of intact GAGs on the cell surface was tested using proteoglycan-degrading enzymes. MM6 cells were preincubated with chondroitinase ABC (0.5 U/mL) for one hour (empty bars in C) and perfused on activated HUVECs. The number of firmly adherent monocytes was determined after accumulation for 5 minutes. Data represent mean ± SEM of at least 3 experiments (*P < .05 as indicated).
Influence of PF4 on the arrest of chemokine-activated monocytes under flow conditions. Monocytic MM6 cells (A) or isolated human blood monocytes (B) were left untreated (control) or stimulated with indicated chemokines (200 ng/mL) (A-B). The relevance of intact GAGs on the cell surface was tested using proteoglycan-degrading enzymes. MM6 cells were preincubated with chondroitinase ABC (0.5 U/mL) for one hour (empty bars in C) and perfused on activated HUVECs. The number of firmly adherent monocytes was determined after accumulation for 5 minutes. Data represent mean ± SEM of at least 3 experiments (*P < .05 as indicated).
To assess whether this effect was dependent on GAGs on the monocyte surface, GAG-degrading enzymes were used. As seen by flow cytometry, MM6 cells expressed chondroitin sulfate as a candidate for surface immobilization of chemokines (not shown). Accordingly, pretreatment of MM6 cells with chondroitinase ABC significantly reduced arrest triggered by RANTES alone or by the combination of PF4 and RANTES (Figure 1C), whereas heparinase I and III had no significant effect (data not shown). This indicates a predominant requirement for chondroitin sulfate in RANTES-induced monocyte arrest.
Increased surface binding of PF4 after coincubation with RANTES
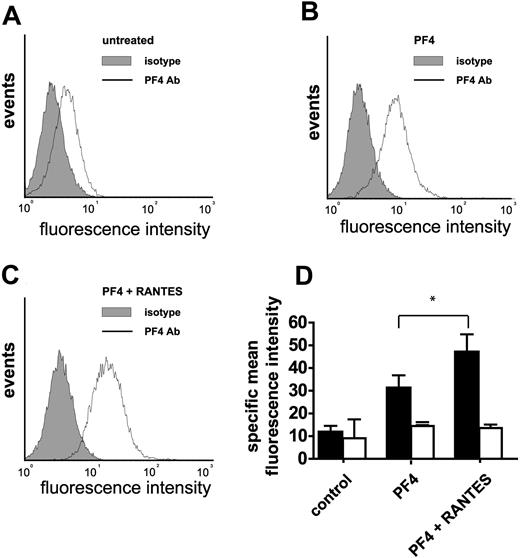
To explore whether enhanced monocyte arrest may be related to direct interactions of PF4 and RANTES, PF4 bound to the cell surface was analyzed by flow cytometry. Preincubation with a combination of RANTES and PF4 resulted in increased binding of PF4 to MM6 cells (Figure 2A-D), possibly attributable to a modulation of PF4 binding affinities or to an availability of additional binding sites following hetero-oligomerization. These results were reproducible with formaldehyde-fixed MM6 cells (not shown), excluding RANTES-induced PF4 secretion or an up-regulation of PF4 binding sites as underlying mechanisms. Pretreatment with chondroitinase ABC inhibited the binding of PF4 to the monocyte surface, indicating that it was mediated by chondroitin sulfate (Figure 2D).
Binding of PF4 to the surface of monocytes with and without coincubation of RANTES. Monocytic MM6 cells were left untreated (A), incubated with PF4 (B), or were coincubated with RANTES and PF4 (C). Chemokines were applied for one hour at room temperature (5 μg/mL), formaldehyde fixed, and subsequently stained with an anti-PF4 antibody (solid line) or isotype control (filled) and analyzed by flow cytometry. MM6 cells were preincubated with chondroitinase ABC (0.5 U/mL) for one hour (empty bars in D). Mean fluorescence of isotype control binding was subtracted from PF4 antibody binding and expressed as mean specific fluorescence (D). Data represent mean ± SEM of 5 independent experiments (*P < .05 vs PF4).
Binding of PF4 to the surface of monocytes with and without coincubation of RANTES. Monocytic MM6 cells were left untreated (A), incubated with PF4 (B), or were coincubated with RANTES and PF4 (C). Chemokines were applied for one hour at room temperature (5 μg/mL), formaldehyde fixed, and subsequently stained with an anti-PF4 antibody (solid line) or isotype control (filled) and analyzed by flow cytometry. MM6 cells were preincubated with chondroitinase ABC (0.5 U/mL) for one hour (empty bars in D). Mean fluorescence of isotype control binding was subtracted from PF4 antibody binding and expressed as mean specific fluorescence (D). Data represent mean ± SEM of 5 independent experiments (*P < .05 vs PF4).
Solid-phase detection of RANTES/PF4 interaction
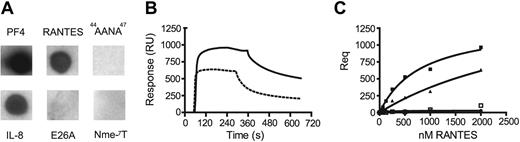
To obtain further evidence for an interaction of PF4 and RANTES, ligand blots were performed. In agreement with findings that PF4 binds IL-8 with high affinity,7 we observed binding of PF4 to immobilized IL-8 (Figure 3A). Notably, we were also able to detect binding of PF4 to immobilized wild-type RANTES. To identify structural properties responsible for the interaction of RANTES with PF4, we used different variants of RANTES, which are defective in higher order oligomerization. Synthetic RANTES Nme-7T is N-methylated at threonine-7 and has been shown to exist strictly as a monomer.22 Glutamic acid-26 mutant E26A RANTES has been shown to occur mainly as a tetramer,32 while 44AANA47 RANTES contains a triple mutation in the principal GAG binding site that impairs GAG binding as well as aggregation.20,21 None of the immobilized oligomerization-defective variants showed binding to PF4 immobilized on nitrocellulose (Figure 3). In order to confirm these results, the interaction between RANTES and PF4 was investigated using surface plasmon resonance. In agreement with the ligand blot (Figure 3A), wild-type RANTES bound to synthetic biotinylated PF4 (Figure 3B). In addition, tetrameric E26A RANTES also bound to bPF4, albeit with lower affinity (Figure 3B-C), while monomeric Nme-7T RANTES and 44AANA47 RANTES did not bind to bPF4 (Figure 3C). RANTES showed considerable background binding (up to 50% of the specific signal) and displayed complex binding behavior. This, in conjunction with the inherent heterogeneity of RANTES, prevented a quantitative kinetic analysis for the interaction between RANTES and PF4. Apparent equilibrium dissociation constants were derived for the interaction between PF4 with RANTES or E26A RANTES from equilibrium binding measurements using nonlinear regression and were found to be 0.8 μM and 3.8 μM, respectively. These data suggest that the same residues of RANTES involved in either oligomerization or binding to GAGs may also be a prerequisite for the heterotypic interaction with PF4.
Interaction of PF4 with different chemokines. Chemokines (3 μg) were applied onto a nitrocellulose membrane and incubated with PF4 (1 μg/mL). PF4 was detected by horseradish peroxidase (HRP)–conjugated secondary antibodies and enhanced chemiluminescence. Of 3 comparable experiments, 1 representative is shown (A). Representative surface plasmon resonance (SPR) sensorgrams of RANTES and E26A-RANTES (both 2.5 μM) binding to immobilized biotinylated PF4 on a streptavidin-coated C1-sensorchip (B). RANTES (solid squares), E26A-RANTES (triangles), Nme-7T-RANTES (dots), and 44AANA47 RANTES (open squares) were injected at 0.125, 0.25, 0.5, 1, or 2 μM, and equilibrium responses are shown as a function of concentration. Solid lines represent fits with a single site binding model (C).
Interaction of PF4 with different chemokines. Chemokines (3 μg) were applied onto a nitrocellulose membrane and incubated with PF4 (1 μg/mL). PF4 was detected by horseradish peroxidase (HRP)–conjugated secondary antibodies and enhanced chemiluminescence. Of 3 comparable experiments, 1 representative is shown (A). Representative surface plasmon resonance (SPR) sensorgrams of RANTES and E26A-RANTES (both 2.5 μM) binding to immobilized biotinylated PF4 on a streptavidin-coated C1-sensorchip (B). RANTES (solid squares), E26A-RANTES (triangles), Nme-7T-RANTES (dots), and 44AANA47 RANTES (open squares) were injected at 0.125, 0.25, 0.5, 1, or 2 μM, and equilibrium responses are shown as a function of concentration. Solid lines represent fits with a single site binding model (C).
Functional interactions with PF4 are not supported by E26A RANTES
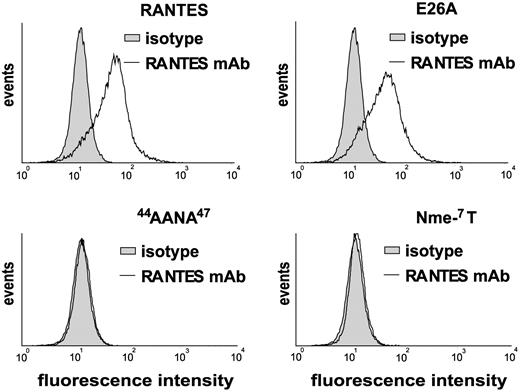
Since the RANTES variants used may differ not only in their binding to PF4 but also in their binding to GAGs,20,22 we studied cell surface binding following preincubation of MM6 cells. The 44AANA47 mutant and the Nme-7T-variant were hardly detectable on the monocyte surface (Figure 4). In contrast, E26A RANTES and wild-type RANTES showed robust and equivalent binding to monocytic cells (Figure 4).
Surface binding of RANTES and its variants on monocytes. Monocytic MM6 cells were incubated for 60 minutes at room temperature with RANTES or the indicated variants (all 1 μg/mL) and analyzed by flow cytometry. Of 4 comparable experiments, 1 representative is shown.
Surface binding of RANTES and its variants on monocytes. Monocytic MM6 cells were incubated for 60 minutes at room temperature with RANTES or the indicated variants (all 1 μg/mL) and analyzed by flow cytometry. Of 4 comparable experiments, 1 representative is shown.
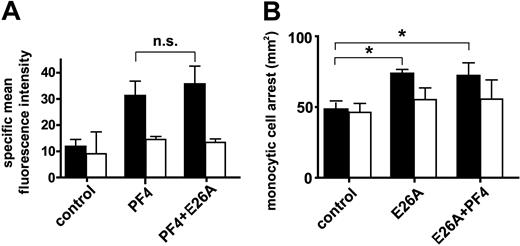
Because E26A RANTES was the only mutant exhibiting impaired heterophilic interactions with PF4 and at the same time preserved surface binding to monocytic cells, we next tested whether it also affects surface binding of PF4, as seen in Figure 2B, and whether it allows enhancement of arrest by PF4. In contrast to coincubation with wild-type RANTES, however, a significant increase in PF4 surface binding was not observed after combination with E26A RANTES (Figure 5A). In line with previous findings,33 E26A RANTES was immobilized on HUVECs (data not shown) and retained an ability to trigger monocyte arrest under flow conditions almost equivalent to that of wild-type RANTES (Figure 5B). Pretreatment with chondroitinase ABC confirmed that PF4 binding and E26A-triggered arrest involved chondroitin sulfate (Figure 5A-B). In contrast to the combination of wild-type RANTES and PF4, the combination of E26A RANTES with PF4 failed to further enhance monocyte arrest (Figure 5B). Thus, the heterophilic interaction with PF4, which is attenuated by mutation of the E26 residue in RANTES, is required for the functional enhancement of RANTES-mediated arrest by PF4. This clearly indicates that a direct interaction of RANTES and PF4 is necessary for increased arrest.
Characterization of functional effects of E26A-RANTES. Surface binding of PF4 was analyzed by flow cytometry in monocytic MM6 cells pretreated with PF4 alone or in combination with E26A-RANTES (A). MM6 cells stimulated with E26A-RANTES alone or in combination with PF4 (200 ng/mL each) were perfused on activated HUVECs. The number of firmly adherent monocytes was determined after accumulation for 5 minutes (B). MM6 cells were preincubated with chondroitinase ABC (0.5 U/mL) for one hour (empty bars). Data represent mean ± SEM of 4 independent experiments (*P < .05 or not significant as indicated).
Characterization of functional effects of E26A-RANTES. Surface binding of PF4 was analyzed by flow cytometry in monocytic MM6 cells pretreated with PF4 alone or in combination with E26A-RANTES (A). MM6 cells stimulated with E26A-RANTES alone or in combination with PF4 (200 ng/mL each) were perfused on activated HUVECs. The number of firmly adherent monocytes was determined after accumulation for 5 minutes (B). MM6 cells were preincubated with chondroitinase ABC (0.5 U/mL) for one hour (empty bars). Data represent mean ± SEM of 4 independent experiments (*P < .05 or not significant as indicated).
PF4 and RANTES from activated platelets
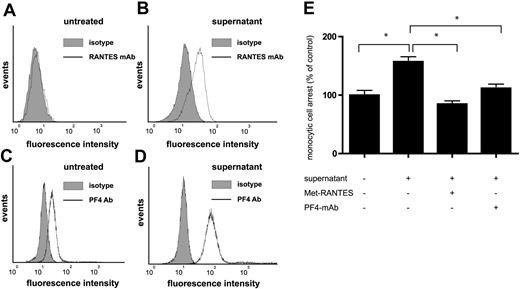
Finally, we tested whether the effects of platelet supernatants on monocyte arrest are attributable to the mechanisms defined for recombinant RANTES and PF4 herein. In line with findings that RANTES and PF4 released from activated platelets may bind to the monocyte surface,18 flow cytometric analysis revealed that the incubation of MM6 cells with supernatants of thrombin-activated platelets resulted in a marked increase in surface binding of both PF4 and RANTES (Figure 6A-D). The stimulation of monocytic cells with supernatants of activated platelets further resulted in a significant enhancement of their arrest on activated HUVECs in flow, which was blocked by pretreatment with Met-RANTES and partially reduced with polyclonal antibodies against PF4 (Figure 6E). Since human PF4 itself does not trigger monocyte arrest, this indicates that PF4 in platelet supernatants may support or facilitate RANTES-triggered monocyte arrest.
Involvement of PF4 and RANTES in monocyte arrest stimulated by platelet supernatants. Monocytic MM6 cells were left untreated (A,C) or incubated with supernatants of human platelets (106/μL) activated with thrombin (0.5 U/mL) for 5 minutes (B,D). The surface binding of RANTES (A,C) and PF4 (B,D) was analyzed by flow cytometry in comparison with isotype control (filled histograms). MM6 cells stimulated with platelet supernatants for 5 minutes and perfused on activated HUVECs, and the number of firmly adherent monocytes was determined after accumulation for 5 minutes (E). Data represent mean ± SEM of 4 independent experiments (*P < .05 as indicated).
Involvement of PF4 and RANTES in monocyte arrest stimulated by platelet supernatants. Monocytic MM6 cells were left untreated (A,C) or incubated with supernatants of human platelets (106/μL) activated with thrombin (0.5 U/mL) for 5 minutes (B,D). The surface binding of RANTES (A,C) and PF4 (B,D) was analyzed by flow cytometry in comparison with isotype control (filled histograms). MM6 cells stimulated with platelet supernatants for 5 minutes and perfused on activated HUVECs, and the number of firmly adherent monocytes was determined after accumulation for 5 minutes (E). Data represent mean ± SEM of 4 independent experiments (*P < .05 as indicated).
Discussion
PF4 has been shown to modulate the biologic functions of multiple proteins (eg, bFGF-2, VEGF165, or protein C) by heterophilic interaction.25,27,34 Here we demonstrate that similar mechanisms may account for the differential effects of PF4 on the proadhesive activities of CC- and CXC-chemokines. PF4 alone does not promote adhesion, but abrogates the arrest of monocytes triggered by IL-8 on HUVECs in shear flow, in accordance with its effect on IL-8 signaling.7 In contrast, RANTES-triggered arrest of monocytes was enhanced by heterophilic interactions with PF4.
Glycosaminoglycans, such as chondroitin sulfate, play an important role in the induction of monocyte arrest by RANTES, as shown by the neutralizing effect of soluble chondroitin sulfate.35 Moreover, treatment with heparitinase inhibited monocyte arrest mediated by GRO-α on activated HUVECs.28 Similarly, the presence of chondroitin sulfate appeared to be crucial for the synergistic effect on monocyte arrest stimulated by the combination of RANTES and PF4, as revealed by enzymatic cleavage with chondroitinase ABC. Although both RANTES and PF4 bind to heparin as well as other GAGs, heparinase treatment did not significantly affect this increase in monocyte arrest. Thus, GAGs may form a highly specific substrate on cell surfaces that allows selective chemokine presentation and subsequent activation of their cognate receptors.
Several mechanisms, which may not be mutually exclusive, can be proposed to provide an explanation for the increased arrest triggered by the combination of PF4 and RANTES. Firstly, PF4 may form hetero-oligomers with RANTES, which may promote binding of RANTES to GAGs on monocytes by recruiting additional binding sites or by altering affinity to enhance chemokine-receptor activation. In turn, the presence of RANTES may increase the binding of PF4 to the monocyte surface by conformational changes enhancing the affinity of PF4 for GAGs. This may be due to a distinctive interface formed by the hetero-oligomer, which may bind to both RANTES-specific and PF4-specific GAGs. Given the moderate affinity of RANTES to PF4 (apparent dissociation constant [Kd] = 0.8 μM), specific GAG substrates may further facilitate the interaction of RANTES and PF4 owing to mechanisms resembling cooperativity. Notably, the E26A RANTES mutant, which has impaired higher order oligomerization and PF4 interaction, but retains affinity for heparin and cell surface binding, did not increase binding of PF4 to monocytes, suggesting an important role for this residue in hetero-oligomerization and its functional consequences. Alternatively, higher order oligomerization of RANTES may be a prerequisite for the interaction with PF4. Although the tetrameric structure of E26A RANTES appeared to retain a limited capacity for interaction with PF4 (apparent Kd = 3.8 μM), the low affinity of this interaction may not be sufficient to support RANTES functions. Importantly, monocyte arrest triggered by E26A RANTES was not influenced by PF4, implying that higher order hetero-oligomerization plays a key role in the enhancement of monocyte arrest by PF4.
Secondly, chemokine complexes composed of PF4 and RANTES may induce or stabilize heterodimerization of chemokine receptors resulting in different signaling events. Similar to other receptors (eg, CCR2 and CXCR4) undergoing ligand-induced heterodimerization, CCR5 oligomers are present in unstimulated cells, and RANTES induces and stabilizes the oligomeric conformation with the highest binding affinity.36-39 Conversely, the monomeric conformation of CCR5 is an inactive receptor state, unable to trigger cell functions or to activate the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway. Moreover, CCR5 has been shown to heterodimerize with CCR2, and the resulting receptor complex activates signaling events distinct from those triggered by the homodimers.40
Thirdly, PF4 might induce intracellular signaling, leading to enhanced RANTES-dependent effects. Neither PF4 nor tumor necrosis factor α (TNF-α) alone but their combination induces a relevant exocytotic response by neutrophils, suggesting the participation of more than one signaling pathway controlling this process.41 Indeed, neutrophil exocytosis induced by PF4 and TNF-α is regulated by at least 3 different signaling elements: p38 mitogen-activated protein (MAP) kinase, phosphatidylinositol 3 (PI 3) kinase, and Lyn src-kinase.42 Similar to TNF-α, RANTES is able to activate the p38 MAP kinase pathway in monocytes via CCR5,43,44 indicating the possibility of an analogous signaling mechanism for the costimulatory effect of RANTES and PF4.
The latter considerations may also be important for the abrogation of IL-8 signaling by PF4 in CD34+ progenitors, which is unlikely due to direct competition of PF4 for the IL-8 receptors CXCR1 or CXCR2, because PF4 neither displaces IL-8 bound to neutrophils41 nor down-regulates CXCR1/CXCR2 expression.45 The effect may rather be attributable to an activation of CXCR3b or a different specific high-affinity CXC-receptor that may generate an intracellular signal that, in turn, blocks downstream IL-8 signaling. Such mechanisms are supported by findings that PF4 blocks bFGF2-dependent phosphorylation of external signal-regulated kinase (ERK) but not AKT in ECs at concentrations that do not impair FGF2 binding to its receptors.34
Contrary functional effects of PF4 at different concentrations5 modulating the activity of IL-8 highlight the relevance for oligomerization for the activity of chemokines. At concentrations of 0.1 to 1 ng/mL, PF4 occurs predominantly as a monomer46 and acts synergistically with IL-8 in suppressing myeloid progenitor cell proliferation.5 At high concentrations, however, PF4 forms a tetramer and abrogates IL-8–induced signaling,7 as well as IL-8–triggered arrest of human monocytes, as shown herein. The differential effects of PF4 on the function of distinct chemokines (eg, enhancing RANTES activity and suppressing IL-8 signals) are paralleled by recent findings in neutrophils.45 At substimulatory concentrations, PF4 drastically reduced neutrophil adhesion to endothelium in response to connective tissue–activating peptide III (CTAP III), while neutrophil-activating peptide 2 (NAP-2)–mediated adhesion was significantly enhanced, in line with the results on RANTES-triggered monocyte arrest.
Activation of platelets leads to secretion of proinflammatory mediators and chemokines and plays a crucial role in inflammatory diseases, atherosclerosis, and neointima formation.17,18,47,48 Both RANTES and PF4 are released from α-granules upon platelet stimulation and can be deposited on inflamed or atherosclerotic endothelium and monocytes.18 Low concentrations (< 1 nM) of PF4 and RANTES have been detected in human plasma. However, a 100- to 1000-fold increase in their concentrations has been observed after platelet activation.11,49 As both chemokines are stored in the same compartment, the formation of heteroaggregates is likely to also occur in vivo. Our results show that both chemokines bind to the surface of monocytes and are involved in mediating increased monocyte arrest on inflamed endothelium following platelet perfusion. Given that PF4 alone does not affect arrest, these data indicate that the amplifying mechanism by PF4 is also operative following platelet activation and the release of multiple platelet-derived mediators, and consequently in promoting atherogenic recruitment of monocytes.
Taken together, our findings support the notion that hetero-aggregates of PF4 and RANTES crucially contribute to the surface binding and the concerted activity of platelet-derived chemokines, in triggering monocyte recruitment in inflammation or atherosclerosis.
Prepublished online as Blood First Edition Paper, September 30, 2004; DOI 10.1182/blood-2004-06-2475.
Supported by grants of the Deutsche Forschungsgemeinschaft WE1913/2-3 and WE1913/5-1 to C.W.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal