Comment on Marcus et al, page 1417
Rituximab is commonly administered by itself or with chemotherapy in patients with follicular lymphoma. Now we have randomized data to support the use of rituximab and CVP in untreated follicular lymphoma with unfavorable prognostic features.
Follicular lymphoma is a chronic disease with a variable course; many patients can be monitored for years without therapy yet others need to be treated aggressively at the onset. Median survival in 2004 approaches 14 years, but the disease remains incurable in the absence of an allotransplant. There is also little evidence that grade impacts outcome.1
The long natural history of the disease obscures the fact that for individual patients, outcomes vary significantly. The recently reported follicular lymphoma international prognostic index (FLIPI), based on 5 clinical and laboratory factors, separates patients with follicular lymphoma into 3 approximately equal-sized groups with median overall survival at 10 years ranging from 36% to 71%.2 Going forward, the lens of the FLIPI will help clarify the results of clinical trials in the same way the international prognostic index (IPI) has for trials involving diffuse large B-cell lymphoma (DLBCL) while avoiding the pitfalls of phase 2 trials with subtle selection biases.FIG1
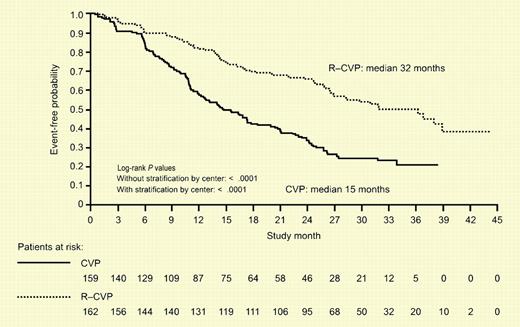
Time to disease progression, relapse, or death after a median follow-up of 30 months among 321 patients assigned to chemotherapy with CVP or with R-CVP. See the complete figure in the article beginning on page 1417.
Time to disease progression, relapse, or death after a median follow-up of 30 months among 321 patients assigned to chemotherapy with CVP or with R-CVP. See the complete figure in the article beginning on page 1417.
In the United States, for patients not enrolled on a clinical trial, rituximab as a single agent or combined with chemotherapy has become standard therapy for follicular lymphoma. There is no randomized data to support this approach but it is clear from the many reported phase 2 studies that the addition of rituximab to chemotherapy is well tolerated with minimal additional side effects largely limited to infusional toxicity and some increased neutropenia.
In this issue, Marcus and colleagues present the results of a random assignment trial of CVP (cyclophosphamide, vincristine, and prednisone) versus rituximab and CVP in untreated patients with follicular lymphoma grades 1-3. This multinational phase 3 study clearly shows that adding rituximab to CVP improved all the primary end points of the study except overall survival. One of the striking features of this study is the exceptionally short event-free survival in both the control and investigation arm. However, the nature of the patient population based on the FLIPI may in part explain the result. Nearly 50% of the patients enrolled had high-risk disease according to the FLIPI. Another explanation of the poor median duration of response in the CVP arm is the modest dosing of cyclophosphamide compared with the typical schema used in the United States where the dose is administered orally at 400 mg/m2/day for 5 days.
We now have a benchmark: median duration of response of 35 months in untreated patients with high-risk FLIPI follicular lymphoma. At the annual meeting of The American Society of Clinical Oncology (ASCO) in 2004, the Eastern Cooperative Oncology Group (ECOG) reported results of maintenance rituximab following CVP chemotherapy, demonstrating a substantial benefit for the maintenance arm.3 However, it is unclear if the addition of maintenance rituximab to a rituximab-based initial therapy has any value in indolent lymphoma. Furthermore, the most pervasive treatment in the United States is rituximab combined with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) based on a small phase 2 study reported by Czuczman et al.4 Thus, a randomized clinical trial controlled for prognostic factors comparing R-CVP and R-CHOP with or without maintenance (4-arm trial) would be valuable. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal