Comment on Spina et al, page 1891
Treatment of HIV-associated non-Hodgkin lymphoma with rituximab plus infusional cyclophosphamide, doxorubicin, and etoposide resulted in high complete remission and 2-year failure-free and overall survival rates but a high rate of infection.
The outlook for patients with HIV-associated non-Hodgkin lymphomas (NHLs) has dramatically improved in the era of highly active antiretroviral therapy (HAART) probably due in large part to improvements in immune status and bone marrow function. In a large trial conducted prior to the HAART era comparing low-dose methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone (m-BACOD) with standard-dose m-BACOD, the median survivals in both arms were approximately 8 months, and less than 20% of the patients were long-term survivors.1 A phase 2 trial of infusional chemotherapy with cyclophosphamide, doxorubicin, and etoposide (CDE) for patients with HIV-associated NHL was conducted by the Eastern Cooperative Oncology Group. Complete remission (CR) rates were similar for patients who received HAART (44%) and those who did not (47%). At a median follow-up time of 50 months, 47% of the patients receiving HAART, and at a median follow-up time of 78 months, 30% of those not receiving HAART, were alive. In the group that received HAART no deaths were due to treatment, while treatment-related mortality was 9% among those who did not receive HAART.2 FIG1
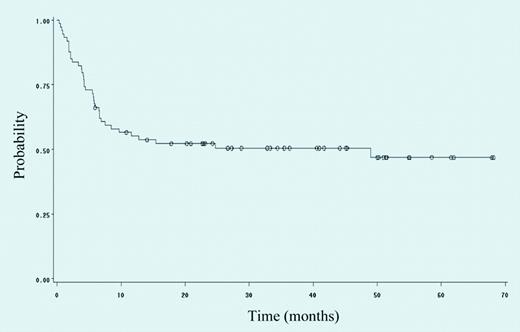
Event-free survival of 74 patients with HIV-NHL treated with rituximab plus infusional cyclophosphamide, doxorubicin, and etoposide. See the complete figure in the article beginning on page 1891.
Event-free survival of 74 patients with HIV-NHL treated with rituximab plus infusional cyclophosphamide, doxorubicin, and etoposide. See the complete figure in the article beginning on page 1891.
The addition of rituximab to standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) has improved the outlook for patients with diffuse large B-cell lymphoma (DLBCL) without HIV infection.3 However, in the HIV setting, in which 60% to 70% of lymphomas are DLBCL, addition of rituximab to CHOP in a randomized trial of the AIDS Malignancy Consortium (AMC) did not result in an improved response rate. Significantly, more grade 3/4 neutropenia was seen in the rituximab group (39%) compared with the group that did not receive rituximab (17%). Deaths due to infection occurred in 7% of the rituximab-treated group and 2% of the group who did not receive rituximab.4
In the present issue of Blood, Spina and colleagues added rituximab to infusional CDE in patients with HIV-associated NHL and report a high CR rate (70%) and 2-year event-free and overall survival rates of 59% and 64%, respectively. The 2-year event-free survival rate was lower (52%; Figure 1), reflecting toxicity. Serious infections were seen in 47% of patients, including opportunistic infections in 14%. Mortality due to infection was 8%.
Although the follow-up is short in the present trial, similar results were reported with an infusional regimen of etoposide, prednisone, vincristine, and doxorubicin followed by dose-adjusted cyclophosphamide according to initial CD4 count and level of neutropenia during treatment (DA-EPOCH) without the addition of rituximab. The median follow-up time in that study was approximately 4 years.5
The AMC is currently combining DA-EPOCH with concurrent or sequential rituximab in a randomized phase 2 trial for HIV-associated lymphoma. The trial is ongoing. Clearly, the improvements in immune and bone marrow function with HAART have made more aggressive treatment of HIV-associated lymphoma possible. Whether this is due to the possibility of increased drug-dose intensity or continuous drug exposure to the tumor by infusion is unclear. For the present, it is probably safest to avoid use of rituximab with chemotherapy for HIV-associated lymphoma outside the setting of a clinical trial. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal