Abstract
Prevention of autoimmune diabetes and induction of islet transplantation tolerance in nonobese diabetic (NOD) mice can be reached by induction of mixed chimerism via bone marrow transplantation (BMT), but this procedure requires total body irradiation (TBI) conditioning of the recipients. The toxicity of radiation and potential for graft-versus-host disease (GVHD) prevents its clinical application. Donor CD8+ T cells play a critical role in facilitation of engraftment but also contribute to induction of GVHD in TBI-conditioned recipients. Here, we showed that high doses of donor CD8+ T cells in combination with bone marrow (BM) cells induced mixed chimerism without GVHD in NOD recipients conditioned with anti-CD3 monoclonal antibody (mAb). The prevention of GVHD in those recipients was associated with low-level production of inflammatory cytokines (ie, tumor necrosis factor α [TNF-α]), high-level production of anti-inflammatory cytokines (ie, interleukin 4 [IL-4] and IL-10), and confining of the donor CD8+ T-cell expansion to lymphohematopoietic tissues. The chimeric NOD recipients showed donor-specific tolerance and reversal of insulitis. These results demonstrate that donor CD8+ T-cell–mediated facilitation of engraftment can be separated from GVHD in nonirradiated recipients. This regimen may have potential application in the treatment of autoimmune disorders as well as induction of transplantation tolerance.
Introduction
Type 1 diabetes is an autoimmune disease characterized by destruction of insulin-secreting pancreatic islet β cells by pathogenic autoreactive T cells.1,2 By most accounts, the nonobese diabetic (NOD) mouse represents an ideal animal model for human type 1 diabetes.3 Female NOD mice develop insulitis at about 4 weeks of age and begin to show diabetes from about 15 weeks of age.3 Induction of mixed chimerism via transplantation of bone marrow (BM) cells from nonautoimmune donors into autoimmune NOD mice has been shown to reverse insulitis and prevent the development of diabetes and induce tolerance to donor islet cells.4-8 However, the bone marrow transplantation (BMT) procedures require nonmyeloablative total body irradiation (TBI) conditioning of the recipients.4-8 The toxicity of TBI conditioning and potential for graft-versus-host disease (GVHD) prevents the application of BMT to treating type 1 diabetes and the induction of immune tolerance for islet cell transplantation.9 This underlies the need for a radiation-free regimen. However, a radiation-free regimen that induces mixed chimerism in autoimmune mice has not been described, although administration of costimulatory blockade (anti-CD40L) has been reported to induce mixed chimerism in nonautoimmune mice.10,11
GVHD in TBI-conditioned recipients is caused by both TBI-conditioning procedures and donor T-cell attack of host epithelial tissues such as gut, skin, and liver.12 TBI conditioning plays a critical role in initiating the tissue damage and inflammatory cascade.13-15 The TBI-damaged host tissues release inflammatory cytokines (ie, tumor necrosis factor α [TNF-α], interleukin 6 [IL-6], and IL-1) and chemokines. Additionally, the gut tissue damage caused by TBI allows the release of lipopolysaccharide (LPS) from intestinal flora, which induces a wide range of secondary inflammatory actions. The inflammatory cytokines and chemokines induce the maturation and activation of host antigen-presenting cells (APCs), and the activated host APCs activate donor T cells.16 The activated donor T cells up-regulate chemokine receptors in response to inflammatory chemokines and cytokines and migrate to inflammatory epithelial tissues and differentiate into Type-1 T helper (Th1) and Type-2 cytotoxic T (Tc1) cells. The Th1 cells release inflammatory cytokines (such as interferon γ [IFN-γ] and TNF-α) that further enhance local inflammation, and the Tc1 cells attack the host tissues so that GVHD occurs.14
GVHD in TBI-conditioned recipients can be prevented by depletion of donor T cells, but depletion of donor T cells results in a marked increase in engraftment failure.17 Donor CD8+ T cells play a critical role in facilitating donor stem cell engraftment in both murine and human BM transplant recipients, although they contribute to GVHD induction in TBI-conditioned recipients.18-20 It was previously reported that donor T-cell infusion after the waning of inflammatory responses induced by TBI conditioning converted mixed chimerism into complete chimerism without causing GVHD and that donor CD8+ T cells play a critical role in this conversion.21-23 Therefore, in the current study we tested our hypothesis that donor CD8+ T cells can facilitate donor stem cell engraftment without causing GVHD in nonirradiated recipients. We observed that injections of high doses of donor CD8+ T cells in combination with donor BM cells induced stable mixed chimerism without GVHD in nonirradiated NOD mice preconditioned with anti-CD3 monoclonal antibody (mAb). The prevention of GVHD in anti-CD3–conditioned NOD recipients was associated with low-level production of inflammatory cytokines (ie, TNF-α), high-level production of anti-inflammatory cytokines (ie, IL-4 and IL-10), and confining of the donor CD8+ T-cell expansion to lymphohematopoietic tissues. In addition, the chimeric NOD recipients showed donor-specific tolerance, reversal of insulitis, and resistance to diabetes development.
Materials and methods
Mice
Female NOD/LtJ (H-2g7), FVB/N (H-2q), B10A(H-2a), C57BL/6 (H-2b), and BALB/c (H-2d) mice at 6 to 8 weeks of age were purchased from The Jackson laboratory (Bar Harbor, ME) and maintained in a pathogen-free room at City of Hope Research Animal Facilities (Duarte, CA). Mice at an age of 8 to 12 weeks were used in the current studies.
Monoclonal antibodies, flow cytometric analysis, and cell sorting
Anti-CD3 mAb (145-2C11) hybridomas were purchased from American Type Culture Collection (ATCC; Manassas, VA). Anti-CD3 mAbs were purified from the culture supernatant using protein G columns as described previously.24,25 The fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, allophycocyanin (APC)–, or cyanin 7 (Cy7)–allophycocyanin (CYT-APC)–conjugated mAbs to mouse T-cell receptor αβ (TCRαβ), B220, Mac-1, Gr-1, H-2q, H-2b, and H-2d were all purchased from BD Pharmingen (San Diego, CA). Multiple-color fluorescence-activated cell sorter (FACS) analysis and sorting were performed at City of Hope FACS facility using a 4-laser MOFLO immunocytometry system (Dako Cytomation, Fort Collins, CO), and data were analyzed using FLOWJO software (Tree Star, San Carlos, CA), as described previously.25 CD8+ and CD4+ T cells were purified with anti-CD8–FITC or anti-CD4–FITC and anti-FITC microbeads using a magnetic purification system from Miltenyi Biotec (Auburn, CA). The purity of positively selected CD8+ or CD4+ T cells was greater than 96%.
Anti-CD3 mAb treatment of mice and BMT
NOD/LtJ, C57BL/6, and BALB/c mice (female, 8-12 weeks old) were injected intravenously with anti-CD3 mAb (145-2C11) at a dose of 500 μg/mouse. One week after antibody injection, mice were given 1 or 2 intravenous injections of donor BM (100 × 106 to 200 × 106/injection) in combination with donor CD8+ T cells (5 × 106 to 20 × 106/injection). There was a 1-week interval between 2 injections of donor cells. The recipients were monitored for clinical signs of GVHD as described previously.18,26
Skin grafting
A full-thickness skin graft (1 × 1.5 cm2) was harvested from the dorsal wall of a donor, placed onto the graft bed on a recipient's left or right back, and covered with Vaseline gauze and protective tape. Grafts were inspected on day 7, then daily for the first month, and 1 time per week thereafter. Grafts were considered rejected at the time of complete sloughing or formation of a dry scab.
Mixed lymphocyte reaction
Responder cells (0.2 × 106/well) were cocultured with stimulator cells (0.5 × 106/well, irradiated with 30 Gy [3000 rad]) in 10% fetal calf serum (FCS) complete RPMI medium for 5 days. Sixteen to 18 hours before harvesting, the cells were pulsed with 3H thymidine (1 μCi/well [0.037 MBq/well]). The stimulation index was calculated by dividing mean counts per minute (cpm) from responses against host, donor, or third-party by mean background cpm (responder only in culture).
Histopathology of skin, small intestine, and pancreatic islets and immunofluorescence microscopy
Histopathologic specimens from skin, small intestine, and pancreata of NOD mice and chimeric NOD recipients were fixed in formalin before embedding in paraffin blocks. Tissue sections were stained with hematoxylin and eosin. Insulin staining was done using Tech-mate 1000 autostainer (Ventana, Tucson, AZ). Double immunofluorescent labeling was performed on 5-μm–thick cryostat sections from snap-frozen pancreatic tissues. The staining procedures were described previously.27 The samples were visualized with an Olympus BX51 fluorescent microscope, equipped with Olympus 20 ×/0.70 and 40 ×/0.90 Planapo objectives (Olympus America, Melville, NY) and a Pixera (600CL) cooled CCD camera (Pixera, Los Gatos, CA). Fluorescent images relative to each marker were collected using a corresponding filter set and Pixera viewfinder acquisition software 3.0. Color composite images were generated using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Measurement of cytokines in serum and culture supernatants
Sera were harvested on days 0, 3, and 5 after BMT. Culture supernatants were from a 48-hour culture of 0.5 × 106 mononuclear cells from spleen, lymph node (LN), or liver. The culture cells were stimulated with plate-bound anti-CD3 mAb (145-2C11) and 5 μg/mL soluble anti-CD28 (37.51; BD Pharmingen). Cytokines were measured using the Luminex Lab MAP system and enzyme-linked immunosorbent assay (ELISA) kits (Biosource International, Camarillo, CA) as described previously.18,28
Statistical analysis
Time to graft rejection or time to diabetes onset among groups was compared using the log-rank test with program GraphPad Prism Version 3.0 (Graph Pad Software, San Diego, CA). Comparison of 2 means was analyzed using unpaired 2-tail Student t test.
Results
High doses of donor CD8+ T cells in combination with donor BM cells induced stable long-term mixed chimerism in NOD recipients conditioned with anti-CD3 mAb
Recent studies have shown that irradiation itself plays a critical role in the induction of GVHD and that higher doses of irradiation are associated with more severe GVHD.13-15 In the current study, we tested whether donor CD8+ T cells facilitate the induction of chimerism without GVHD in nonirradiated autoimmune NOD mice.
NOD mice (female, 8-12 weeks old) were conditioned with 1 intravenous injection of anti-CD3 mAb (145-2C11) at a dose of 500 μg/mouse. TCRαβ+ T cells in all tissues including blood, spleen, LN, liver, BM, and thymus were depleted 1 week after anti-CD3 treatment, partially recovered by 2 weeks, and completely recovered to pretreatment levels by 3 to 4 weeks (data not shown). As a result, we considered 7 days following antibody treatment would represent the optimal time for donor cell infusion.
Hence, 7 days after anti-CD3 injection, NOD mice were injected with 200 × 106 BM cells alone or in combination with 20 × 106 spleen CD8+ T cells from FVB/N (H-2q) donors. Thereafter, the recipients were checked for chimerism by flow cytometric analysis of donor-type H-2q+ cells in peripheral blood. The recipients given donor BM or BM in combination with CD8+ T cells showed low levels (5%-10%) of chimerism with donor cells for the first 4 weeks after cell injection, but that chimerism disappeared by 8 weeks (data not shown). Therefore, a single injection of donor BM or BM with CD8+ T cells resulted in only transient chimerism in NOD recipients conditioned with anti-CD3.
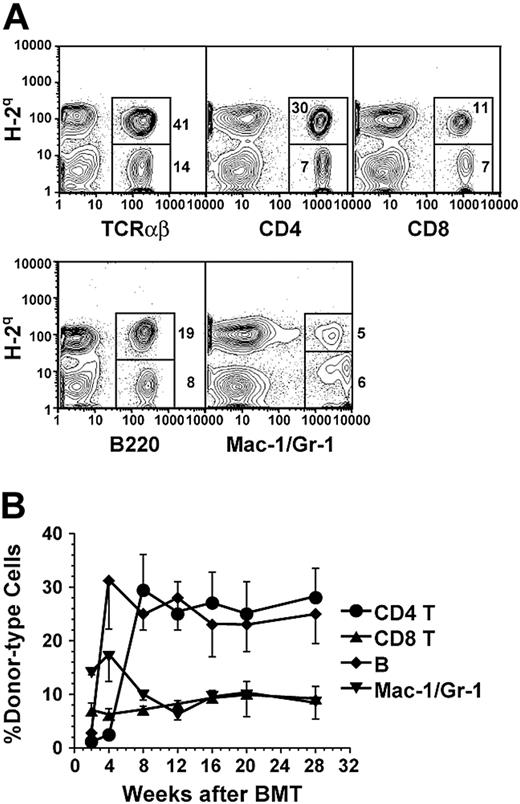
Therefore, in subsequent experiments, the NOD mice were conditioned with anti-CD3 and provided 2 injections of donor BM or 2 injections of donor BM in combination with CD8+ T cells 7 and 14 days after anti-CD3 treatment. Although 2 injections of donor BM alone still resulted in transient chimerism, 2 injections of donor BM with CD8+ T cells resulted in a stable long-term chimerism that lasted for more than 28 weeks after BMT in 90% (18/20) of the NOD recipients (Figure 1; Table 1). The chimerism was measured by staining recipient blood mononuclear cells with anti–donor H-2q versus anti-TCRαβ, -CD4, -CD8, -B220, and -Mac-1/Gr-1 (Figure 1A). The percentage of each subset of donor-type cells among recipient blood mononuclear cells reached a stable level 8 weeks after BMT, with approximately 25% CD4+ T cells, 8% CD8+ T cells, 22% B lymphocytes, and 7% granulocytes/macrophage cells, together totaling about 60% (Figure 1B). The long-term chimeric NOD recipients (24 weeks after BMT) showed mixed chimerism in thymus, blood, spleen, LNs, and BM (data not shown).
Mixed-chimerism in anti-CD3 mAb preconditioned NOD recipients. (A) Flow cytometric analysis of donor-type (H-2q+) cells including TCRαβ+, CD4+, CD8+, B220+, and Mac-1+/Gr-1+ cells in blood mononuclear cells of anti-CD3–conditioned recipients 10 weeks after BMT. The percentage of H-2q+ donor-type and H-2q- host-type cells are shown beside or in the gating boxes. One representative of 12 recipients is shown. (B) Stable multilineage chimerism in peripheral blood of NOD recipients for more than 28 weeks after BMT. ⬡ indicates CD4+; ▴, CD8+;  , B220+; and ▾, Mac-1+/Gr-1+ cells. Values are mean ± SE of 12 recipients combined from 3 experiments (n = 12).
, B220+; and ▾, Mac-1+/Gr-1+ cells. Values are mean ± SE of 12 recipients combined from 3 experiments (n = 12).
Mixed-chimerism in anti-CD3 mAb preconditioned NOD recipients. (A) Flow cytometric analysis of donor-type (H-2q+) cells including TCRαβ+, CD4+, CD8+, B220+, and Mac-1+/Gr-1+ cells in blood mononuclear cells of anti-CD3–conditioned recipients 10 weeks after BMT. The percentage of H-2q+ donor-type and H-2q- host-type cells are shown beside or in the gating boxes. One representative of 12 recipients is shown. (B) Stable multilineage chimerism in peripheral blood of NOD recipients for more than 28 weeks after BMT. ⬡ indicates CD4+; ▴, CD8+;  , B220+; and ▾, Mac-1+/Gr-1+ cells. Values are mean ± SE of 12 recipients combined from 3 experiments (n = 12).
, B220+; and ▾, Mac-1+/Gr-1+ cells. Values are mean ± SE of 12 recipients combined from 3 experiments (n = 12).
Induction of mixed chimerism in anti-CD3-conditioned recipients given different dose of donor CD8+ T and BM cells
Donor . | Donor CD8+ T dose × 106 . | Donor BM dose, × 106 . | Recipient . | % Chimeric recipients . | % Donor-type cells in PBMNCs . |
|---|---|---|---|---|---|
| FVB/N | 20 × 2 | 200 × 2 | NOD | 90% | 43.7-69.6 |
| FVB/N | 10 × 2 | 200 × 1 | NOD | 100% | 39.7-57.5 |
| FVB/N | 5 × 2 | 100 × 1 | NOD | 63% | 34.8-51.4 |
| C57BL/6 | 10 × 2 | 200 × 1 | BALB/c | 100% | 45.6-71.3 |
| B10A | 10 × 2 | 200 × 1 | C57BL/6 | 100% | 50.1-78.1 |
Donor . | Donor CD8+ T dose × 106 . | Donor BM dose, × 106 . | Recipient . | % Chimeric recipients . | % Donor-type cells in PBMNCs . |
|---|---|---|---|---|---|
| FVB/N | 20 × 2 | 200 × 2 | NOD | 90% | 43.7-69.6 |
| FVB/N | 10 × 2 | 200 × 1 | NOD | 100% | 39.7-57.5 |
| FVB/N | 5 × 2 | 100 × 1 | NOD | 63% | 34.8-51.4 |
| C57BL/6 | 10 × 2 | 200 × 1 | BALB/c | 100% | 45.6-71.3 |
| B10A | 10 × 2 | 200 × 1 | C57BL/6 | 100% | 50.1-78.1 |
PBMNCs indicates peripheral blood mononuclear cells.
× 1 or × 2 indicates 1 or 2 injections.
In further experiments, we titrated down the dose of donor CD8+ T and BM cells, and we found that 1 injection of 200 × 106 donor bone marrow cells in combination with 2 injections of 10 × 106 donor CD8+ T cells induced long-term (> 20 weeks) mixed chimerism in all (8/8) NOD recipients (Table 1); furthermore, 1 injection of 100 × 106 donor BM cells in combination with 2 injections of 5 × 106 donor CD8+ T cells induced mixed chimerism in 63% (5/8) of recipients (Table 1). The donor-type cells including T, B, and granulocyte/macrophage cells accounted for more than 35% of total blood mononuclear cells (Table 1).
The induction of mixed chimerism by a combination of donor BM and CD8+ T cells in anti-CD3–conditioned recipients was not dependent on a particular strain combination. C57BL/6 donor cells induced mixed chimerism in 100% (8/8) of BALB/c recipients, and B10A donor cells induced chimerism in all (8/8) C57BL/6 recipients (Table 1).
Donor CD8+ T cells facilitated induction of mixed chimerism without causing GVHD
The major concern regarding infusion of donor CD8+ T cells is GVHD, since donor peripheral CD8+ T cells induce severe GVHD in recipients conditioned with TBI.18,20 Therefore, the chimeric NOD recipients were carefully monitored for the development of GVHD. Interestingly, the chimeric NOD recipients did not show any clinical signs of GVHD (ie, no weight loss, hair loss, or diarrhea), remaining healthy looking with normal body weight over a follow-up period of more than 24 weeks after BMT (Figure 2A-B). In contrast, donor CD8+ T cells induced severe GVHD in TBI-conditioned NOD recipients. As shown in Figure 2A-B, all (8/8) NOD mice conditioned with sublethal TBI (6.5 Gy [650 rad]) and injected with 200 × 106 BM and 20 × 106 CD8+ T cells from FVB/N donors developed severe clinical signs of GVHD including weight loss, hair loss, hunched back, and diarrhea and became moribund 40 to 60 days after BMT.
No signs of GVHD in the anti-CD3–conditioned chimeric recipients. (A) Representatives of GVHD-free recipients conditioned with anti-CD3 and GVHD recipients conditioned with TBI. (B) Body weight change of recipients over a follow-up period of 28 weeks after BMT. ⬡ indicates anti-CD3–conditioned chimeric NOD recipients;  , NOD mice given anti-CD3–conditioning only; and ▾, TBI-conditioned chimeric NOD recipients. Values are mean ± SE of anti-CD3–conditioned chimeric recipients (n = 12), NOD mice given anti-CD3–conditioning only (n = 12), and TBI-conditioned chimeric recipients (n = 8). (C) Histology of skin and small intestine tissues of the anti-CD3–conditioned chimeric recipients (top row), NOD mice given anti-CD3–conditioning only (middle row), and TBI-conditioned chimeric recipients 50 days after BMT (bottom row). One representative of 4 recipients examined in each group is shown.
, NOD mice given anti-CD3–conditioning only; and ▾, TBI-conditioned chimeric NOD recipients. Values are mean ± SE of anti-CD3–conditioned chimeric recipients (n = 12), NOD mice given anti-CD3–conditioning only (n = 12), and TBI-conditioned chimeric recipients (n = 8). (C) Histology of skin and small intestine tissues of the anti-CD3–conditioned chimeric recipients (top row), NOD mice given anti-CD3–conditioning only (middle row), and TBI-conditioned chimeric recipients 50 days after BMT (bottom row). One representative of 4 recipients examined in each group is shown.
No signs of GVHD in the anti-CD3–conditioned chimeric recipients. (A) Representatives of GVHD-free recipients conditioned with anti-CD3 and GVHD recipients conditioned with TBI. (B) Body weight change of recipients over a follow-up period of 28 weeks after BMT. ⬡ indicates anti-CD3–conditioned chimeric NOD recipients;  , NOD mice given anti-CD3–conditioning only; and ▾, TBI-conditioned chimeric NOD recipients. Values are mean ± SE of anti-CD3–conditioned chimeric recipients (n = 12), NOD mice given anti-CD3–conditioning only (n = 12), and TBI-conditioned chimeric recipients (n = 8). (C) Histology of skin and small intestine tissues of the anti-CD3–conditioned chimeric recipients (top row), NOD mice given anti-CD3–conditioning only (middle row), and TBI-conditioned chimeric recipients 50 days after BMT (bottom row). One representative of 4 recipients examined in each group is shown.
, NOD mice given anti-CD3–conditioning only; and ▾, TBI-conditioned chimeric NOD recipients. Values are mean ± SE of anti-CD3–conditioned chimeric recipients (n = 12), NOD mice given anti-CD3–conditioning only (n = 12), and TBI-conditioned chimeric recipients (n = 8). (C) Histology of skin and small intestine tissues of the anti-CD3–conditioned chimeric recipients (top row), NOD mice given anti-CD3–conditioning only (middle row), and TBI-conditioned chimeric recipients 50 days after BMT (bottom row). One representative of 4 recipients examined in each group is shown.
Since TBI-conditioned recipients had the most severe tissue GVHD in skin and gut 40 to 50 days after BMT,18,26 4 of the chimeric NOD recipients were subjected to histologic assessments on day 50 after BMT. No tissue damage was found in the skin and small intestine tissues of the recipients, and no difference was observed between the chimeric recipients and NOD mice treated with anti-CD3 only (Figure 2C). In contrast, 50 days after BMT, the skin tissues of the TBI-conditioned recipients showed hyperplasia in epidermis and lymphocyte infiltration in dermis, while the small intestine tissues showed mucosal atrophy and lymphocyte infiltration (Figure 2C). These results indicate that, in contrast to TBI conditioning, anti-CD3 conditioning prevents GVHD development.
The chimeric NOD recipients showed donor-specific tolerance, reversal of insulitis, and resistance to the development of diabetes
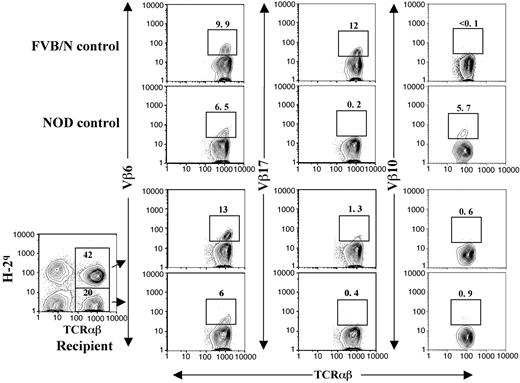
The chimeric NOD recipients were tested for donor-specific tolerance. The recipients received transplants of skin grafts from donor FVB/N and third-party B10A (H-2a) mice 4 to 8 weeks after BMT. All (10/10) donor skin grafts survived for more than 150 days, but the third-party B10A grafts were rejected within 20 days (Figure 3A; P < .001). In addition, the LN cells from the chimeric recipients did not proliferate to stimulation by donor or recipient spleen cells but proliferated vigorously in response to stimulation by third-party B10A spleen cells (Figure 3B). These results indicate that donor- or host-reactive T cells in the chimeric recipients are deleted or unresponsive. Clonal deletion is the major mechanism of tolerance induction in chimeric recipients,29,30 and endogenous superantigen-mediated deletion of TCR Vβ subunits was previously used as an indication of clonal deletion of alloreactive T cells.5,10 Superantigen-mediated clonal deletion was also measured with the long-term chimeric NOD recipients. As shown in Figure 4, Vβ6 and Vβ17 in FVB/N mice were abundant but Vβ10 was deleted. On the other hand, Vβ6 and Vβ10 in NOD mice were abundant but Vβ17 was deleted. In the chimeric NOD recipients, the FVB/N donor-type T (H-2q+TCRαβ+) cells had a 10-fold reduction of Vβ17+ cells compared with FVB/N mice (P < .01) but no reduction of Vβ6+ cells, indicating a clonal deletion of Vβ17+ cells mediated by host NOD superantigens. In contrast, the host-type T (H-2q-TCRαβ+) cells had a 5-fold reduction in Vβ10+ cells (P < .01) but no reduction of Vβ6+ cells, indicating a clonal deletion of Vβ10+ cells mediated by donor FVB/N superantigens. This mutual deletion of donor- and host-type T-cell subsets in chimeric recipients is consistent with previous reports.5,10
Donor-specific tolerance and reversal of insulitis in chimeric NOD recipients. (A) Chimeric NOD mice accepted donor (⬡) but rejected third-party skin grafts (—). (B) Mixed lymphocyte reaction of lymph node cell responders from chimeric recipients at 32 weeks of age against host NOD, donor FVB/N, and third-party B10A spleen cell stimulators. One representative of 3 replicate experiments is shown. (C) Chimeric NOD recipients ( ; n = 12) were resistant to diabetes development compared with control NOD mice without BMT (⬡; n = 26). (D) Histology of pancreata of 3-week-old NOD mice (top row), 8-week-old NOD mice before anti-CD3 treatment (middle row), and 32-week-old chimeric recipients (bottom row). The tissues from each recipient are shown in HE staining (left column), insulin staining (middle column), and 2-color staining of insulin (red), anti-CD3 mAb (green; right column). Severe lymphocyte infiltration is seen within islets of 8-week-old NOD mice, but no infiltration was observed in 3-week-old or 32-week-old chimeric recipients. One representative of 6 mice examined in each group is shown.
; n = 12) were resistant to diabetes development compared with control NOD mice without BMT (⬡; n = 26). (D) Histology of pancreata of 3-week-old NOD mice (top row), 8-week-old NOD mice before anti-CD3 treatment (middle row), and 32-week-old chimeric recipients (bottom row). The tissues from each recipient are shown in HE staining (left column), insulin staining (middle column), and 2-color staining of insulin (red), anti-CD3 mAb (green; right column). Severe lymphocyte infiltration is seen within islets of 8-week-old NOD mice, but no infiltration was observed in 3-week-old or 32-week-old chimeric recipients. One representative of 6 mice examined in each group is shown.
Donor-specific tolerance and reversal of insulitis in chimeric NOD recipients. (A) Chimeric NOD mice accepted donor (⬡) but rejected third-party skin grafts (—). (B) Mixed lymphocyte reaction of lymph node cell responders from chimeric recipients at 32 weeks of age against host NOD, donor FVB/N, and third-party B10A spleen cell stimulators. One representative of 3 replicate experiments is shown. (C) Chimeric NOD recipients ( ; n = 12) were resistant to diabetes development compared with control NOD mice without BMT (⬡; n = 26). (D) Histology of pancreata of 3-week-old NOD mice (top row), 8-week-old NOD mice before anti-CD3 treatment (middle row), and 32-week-old chimeric recipients (bottom row). The tissues from each recipient are shown in HE staining (left column), insulin staining (middle column), and 2-color staining of insulin (red), anti-CD3 mAb (green; right column). Severe lymphocyte infiltration is seen within islets of 8-week-old NOD mice, but no infiltration was observed in 3-week-old or 32-week-old chimeric recipients. One representative of 6 mice examined in each group is shown.
; n = 12) were resistant to diabetes development compared with control NOD mice without BMT (⬡; n = 26). (D) Histology of pancreata of 3-week-old NOD mice (top row), 8-week-old NOD mice before anti-CD3 treatment (middle row), and 32-week-old chimeric recipients (bottom row). The tissues from each recipient are shown in HE staining (left column), insulin staining (middle column), and 2-color staining of insulin (red), anti-CD3 mAb (green; right column). Severe lymphocyte infiltration is seen within islets of 8-week-old NOD mice, but no infiltration was observed in 3-week-old or 32-week-old chimeric recipients. One representative of 6 mice examined in each group is shown.
Clonal deletion of donor- and host-reactive T cells in chimeric NOD recipients. Peripheral blood mononuclear cells (PBMNCs) from control FVB/N and NOD mice were stained with anti-TCRαβ versus anti-Vβ6, Vβ17, or Vβ10. The PBMNCs from long-term (> 24 weeks after BMT) chimeric recipients were stained with anti–H-2q, TCRαβ plus anti-Vβ6, Vβ17, or Vβ10. Donor-type (H-2q+) and host-type (H-2q-) TCRαβ+ cells were gated and then shown in TCRαβ versus Vβ6, Vβ17, or Vβ10. The percentage of H-2q+ and H-2q- TCRαβ+ cells among PBMNCs was shown in the gating boxes. The percentage of Vβ6+, Vβ17+, and Vβ10+ T-cell subsets among total T cells were shown above the gating boxes. One representative of 4 measured recipients is shown.
Clonal deletion of donor- and host-reactive T cells in chimeric NOD recipients. Peripheral blood mononuclear cells (PBMNCs) from control FVB/N and NOD mice were stained with anti-TCRαβ versus anti-Vβ6, Vβ17, or Vβ10. The PBMNCs from long-term (> 24 weeks after BMT) chimeric recipients were stained with anti–H-2q, TCRαβ plus anti-Vβ6, Vβ17, or Vβ10. Donor-type (H-2q+) and host-type (H-2q-) TCRαβ+ cells were gated and then shown in TCRαβ versus Vβ6, Vβ17, or Vβ10. The percentage of H-2q+ and H-2q- TCRαβ+ cells among PBMNCs was shown in the gating boxes. The percentage of Vβ6+, Vβ17+, and Vβ10+ T-cell subsets among total T cells were shown above the gating boxes. One representative of 4 measured recipients is shown.
The chimeric NOD mice were then evaluated for diabetes development. While 89% (23/26) of the control NOD mice given 1 injection of anti-CD3 developed diabetes (blood glucose level > 27.755 mM [500 mg/dL]), none of the chimeric NOD recipients (0/12) developed diabetes (blood glucose level < 8.3265 mM [150 mg/dL]) by the age of 32 weeks (Figure 3C; P < .001). Similar diabetes incidence was found in untreated and anti-CD3–treated NOD mice over an observation period of 32 weeks (ie, 16/20 in the untreated and 23/26 in the anti-CD3–treated at 32 weeks of age). It was reported previously that multiple anti-CD3 treatment of prediabetic NOD mice did not prevent diabetes development, although the same treatment reverses diabetes in overtly diabetic NOD mice.31 In addition, all chimeric recipients (6/6) were free from lymphocyte infiltration in their islets nor was reduction in insulin staining observed. However, all (6/6) control NOD mice at 8 weeks of age before treatment showed severe lymphocyte infiltration (Figure 3D). These results indicate that mixed chimerism in prediabetic NOD mice reverses insulitis and prevents the development of diabetes.
The anti-CD3–conditioned NOD recipients showed low-level production of inflammatory cytokines and high-level production of anti-inflammatory cytokines early after donor CD8+ T-cell injection
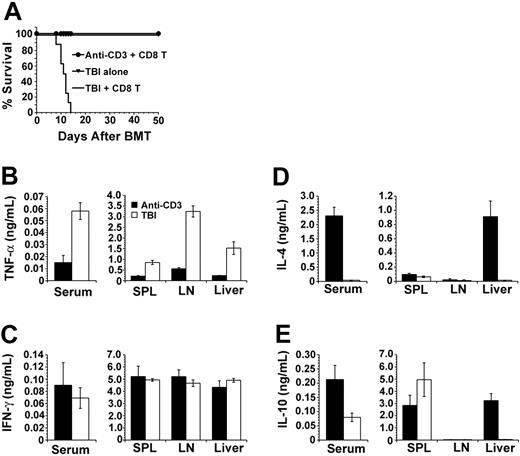
It is a novel observation that high doses of donor CD8+ T cells facilitated donor stem cell engraftment without GVHD in nonirradiated recipients. Next, we studied the mechanisms of GVHD prevention in anti-CD3–conditioned recipients. Inflammatory cytokines (ie, TNF-α) play a critical role in the induction of GVHD in recipients conditioned with TBI,32,33 but IL-4– and IL-10–secreting T and dendritic cells suppress GVHD.18,26,34-36 Thus, we compared the cytokine profiles in serum and culture supernatants of mononuclear cells obtained from spleen, LN, and liver of the TBI- or anti-CD3–conditioned recipients after donor CD8+ T-cell injection. In order to avoid the influence of T cells in donor BM, the recipients were injected with 2 × 106 T-cell–depleted (TCD) BM and 20 × 106 spleen CD8+ T cells from FVB/N donors. First, we observed that all (8/8) TBI-conditioned recipients given donor TCD-BM and CD8+ T cells developed severe clinical signs of GVHD and died within 2 weeks after BMT, although all mice given TBI conditioning alone survived for more than 50 days (Figure 5A). In contrast, all (8/8) anti-CD3–conditioned recipients given donor TCD-BM in combination with CD8+ T cells showed no signs of GVHD and survived for more than 50 days after BMT (Figure 5A).
Comparison of cytokine secretion profile of anti-CD3– or TBI-conditioned recipients. (A) Survival of recipients conditioned with anti-CD3 (⬡) or TBI (—) after injection of donor TCD-BM (2 × 106) and CD8+ T cells (20 × 106). ▾ indicates recipients of TBI alone. There were 8 mice in each group. (B-E) TNF-α, IFN-γ, IL-4, and IL-10 in serum and culture supernatant (anti-CD3, ▪; TBI, □) of recipients from the 2 groups. Values are the mean ± SE of individual recipients in each group (n = 8). SPL indicates spleen.
Comparison of cytokine secretion profile of anti-CD3– or TBI-conditioned recipients. (A) Survival of recipients conditioned with anti-CD3 (⬡) or TBI (—) after injection of donor TCD-BM (2 × 106) and CD8+ T cells (20 × 106). ▾ indicates recipients of TBI alone. There were 8 mice in each group. (B-E) TNF-α, IFN-γ, IL-4, and IL-10 in serum and culture supernatant (anti-CD3, ▪; TBI, □) of recipients from the 2 groups. Values are the mean ± SE of individual recipients in each group (n = 8). SPL indicates spleen.
The sera of the recipients were harvested on days 0, 3, and 5 after BMT. Serum cytokine levels were undetectable on day 0 and peaked on day 5. Compared with TBI-conditioned recipients, the anti-CD3–conditioned recipients had 4-fold lower levels of serum TNF-α (Figure 5B; P < .01) but 50-fold higher IL-4 and 3-fold higher IL-10 levels (Figure 5D-E; P < .01). In addition, mononuclear cells from spleen, LN, and liver of the anti-CD3–conditioned recipients 5 days after BMT secreted 5- to 10-fold lower levels of TNF-α compared with that of TBI-conditioned recipients, and the liver mononuclear cells of the anti-CD3–conditioned recipients secreted more than 20-fold higher levels of IL-4 and IL-10 (Figure 5B,D-E; P < .01). The IFN-γ levels in sera and culture supernatant of the 2 kinds of recipients were similar (Figure 5C; P > .1). These results show that there is low-level production of proinflammatory TNF-α and high-level production of anti-inflammatory IL-4 and IL-10 in the anti-CD3–conditioned recipients early after donor CD8+ T-cell injection.
To identify the source of IL-4 and IL-10 production in liver mononuclear cells from the anti-CD3–conditioned recipients, liver mononuclear cells were analyzed for the percentage of natural killer T (NKT) cells. It is well known that there is a high percentage of NKT cells among T cells in liver, and NKT cells secrete large amounts of IL-4 and IL-10 upon primary stimulation.37,38 As shown in Figure 6, all TCRαβ+ cells among the liver mononuclear cells from the TBI-conditioned recipients were donor type and 95% of them were donor CD8+ T cells. In contrast, there were both donor- and host-type T cells among the liver mononuclear cells from anti-CD3–conditioned recipients, and the percentage and yield of donor T cells was 10-fold lower compared with that of TBI-conditioned recipients (P < .01; Figure 6; Table 2). There were both CD4+ and CD8+ T cells among the residual host T cells, and about 73% of the host CD8- (including CD4+ and CD4-CD8-) T cells were CD1d-αGalCer-tetramer+ T cells. Both donor and host CD8+ T cells were all CD1d-αGalCer-tetramer- (data not shown). These results indicate that host-type NKT cells in the liver of the anti-CD3–conditioned recipients may be the major source of IL-4 and IL-10 production early after BMT. Future studies will reveal the cytokine secretion profile of those NKT cells.
A high percentage of NKT cells among the liver mononuclear cells of the anti-CD3–conditioned recipients. The mononuclear cells from the liver of the anti-CD3– or TBI-conditioned NOD recipients 5 days after BMT were stained with anti–H-2q, anti-TCRαβ, anti-CD8, and CD1d-αGalCer-tetramer. The TCRαβ+ cells were first gated into H-2q+ and H-2q-, then both were shown in TCRαβ versus CD8. The gated TCRαβ+H-2q-CD8- cells were further shown in TCRαβ versus CD1d-αGalCer-tetramer. The percentage of each gated population among total cells was shown inside or beside the gating boxes. One representative of 4 measured recipients is shown. N/A indicates not available.
A high percentage of NKT cells among the liver mononuclear cells of the anti-CD3–conditioned recipients. The mononuclear cells from the liver of the anti-CD3– or TBI-conditioned NOD recipients 5 days after BMT were stained with anti–H-2q, anti-TCRαβ, anti-CD8, and CD1d-αGalCer-tetramer. The TCRαβ+ cells were first gated into H-2q+ and H-2q-, then both were shown in TCRαβ versus CD8. The gated TCRαβ+H-2q-CD8- cells were further shown in TCRαβ versus CD1d-αGalCer-tetramer. The percentage of each gated population among total cells was shown inside or beside the gating boxes. One representative of 4 measured recipients is shown. N/A indicates not available.
Yield of donor CD8+ T cells in the different tissues of the anti-CD3- or TBI-conditioned NOD recipients 5 days after BMT (n = 6, mean ± SE)
Conditioning and recipient tissues . | Yield of mononuclear cells, ×106 . | % Donor-type CD8+ T cells . | Yield of donor-type CD8+ T cells, ×106 . |
|---|---|---|---|
| Anti-CD3 | |||
| Liver | 3.2 ± 0.5 | 5.6 ± 1.1 | 0.17 ± 0.08 |
| Spleen | 282 ± 23 | 4.7 ± 1.5 | 14.5 ± 1.2 |
| Lymph nodes* | 3.6 ± 0.7 | 7.5 ± 2.2 | 0.31 ± 0.11 |
| TBI | |||
| Liver | 2.1 ± 0.8 | 76 ± 4.5 | 1.86 ± 0.12 |
| Spleen | 12 ± 3.2 | 61 ± 8.9 | 8.3 ± 0.7 |
| Lymph nodes | 0.4 ± 0.1 | 71 ± 5.4 | 0.28 ± 0.17 |
Conditioning and recipient tissues . | Yield of mononuclear cells, ×106 . | % Donor-type CD8+ T cells . | Yield of donor-type CD8+ T cells, ×106 . |
|---|---|---|---|
| Anti-CD3 | |||
| Liver | 3.2 ± 0.5 | 5.6 ± 1.1 | 0.17 ± 0.08 |
| Spleen | 282 ± 23 | 4.7 ± 1.5 | 14.5 ± 1.2 |
| Lymph nodes* | 3.6 ± 0.7 | 7.5 ± 2.2 | 0.31 ± 0.11 |
| TBI | |||
| Liver | 2.1 ± 0.8 | 76 ± 4.5 | 1.86 ± 0.12 |
| Spleen | 12 ± 3.2 | 61 ± 8.9 | 8.3 ± 0.7 |
| Lymph nodes | 0.4 ± 0.1 | 71 ± 5.4 | 0.28 ± 0.17 |
Italics indicate marked difference of yield.
The pooled inguinal and mesenteric lymph nodes
Donor CD8+ T cells in anti-CD3–conditioned recipients expanded predominantly in host lymphohematopoietic tissues
The anti-CD3–conditioned recipients given donor CD8+ T and BM cells did not show lymphocyte infiltration in their skin and intestine tissues but the TBI-conditioned recipients given the same dose of donor CD8+ T and BM cells showed heavy lymphocyte infiltration in those tissues (Figure 2C). We thus compared the expansion of the donor CD8+ T cells in the spleen, LNs, and liver of the 2 kinds of recipients. We found that 5 days after BMT, the anti-CD3–conditioned NOD recipients given 20 × 106 CD8+ T cells in combination with 2 × 106 TCD-BM cells showed much larger spleen compared with that of TBI-conditioned recipients given the same dose of donor CD8+ T and BM cells. In addition, the liver of the anti-CD3–conditioned recipients looked normal, but the liver of the TBI-conditioned recipients looked pale and decayed. Histopathology study showed that there was little lymphocyte infiltration in the anti-CD3–conditioned recipient liver tissue but massive lymphocyte infiltration in the TBI-conditioned liver tissue (data not shown). Flow cytometric analysis showed that all TCRαβ+ T cells in spleen, LNs, and liver of TBI-conditioned recipients were donor type, but TCRαβ+ T cells in those tissues of anti-CD3–conditioned recipients contained both donor and host type (Figure 6). Figure 6 shows the pattern of liver mononuclear cells, and the patterns of spleen and LNs were very similar to Figure 6 (data not shown). In addition, the percentage of donor-type T cells in those tissues of TBI-conditioned recipients was 10-fold higher than that of anti-CD3–conditioned recipients (Figure 6; Table 2).
Next, we compared the yield of donor CD8+ T cells in the spleen, LN, and liver from the 2 kinds of recipients. Our previous studies showed that 5 days after BMT, all donor-type T cells in the tissues of recipients were the injected donor T cells.18 As shown in Table 2, the yield of donor CD8+ T cells in the spleen of anti-CD3–conditioned recipients was significantly higher than that of TBI-conditioned recipients (P < .05), due to an about 20-fold increase of spleen mononuclear cells compared with that of TBI-conditioned recipients. In contrast, the yield of CD8+ T cells from the liver of anti-CD3–conditioned recipients was 10-fold less compared with that of TBI-conditioned recipients (P < .01), due to a 10-fold lower percentage of donor CD8+ T cells in the anti-CD3–conditioned recipients than in the TBI-conditioned recipients. The yield of donor CD8+ T cells in the LNs was similar in the 2 kinds of recipients. These results indicate that donor CD8+ T cells expand predominantly in lymphohematopoietic tissues such as spleen and LN in the recipients conditioned with anti-CD3, but they expand in both lymphohematopoietic tissues and GVHD target tissues such as liver, gut, and skin in recipients conditioned with TBI.
Discussion
We have demonstrated here that high doses of donor CD8+ T cells in combination with donor BM cells induced stable permanent mixed chimerism without GVHD in prediabetic NOD mice conditioned with anti-CD3 mAb. The chimeric NOD recipients developed donor-specific tolerance. The chimeric NOD recipients also showed reversal of insulitis and resistance to the development of diabetes.
NOD mice are resistant to tolerance induction, costimulatory blockade regimens failed to induce tolerance in NOD recipients,39 and a radiation-free regimen that can induce mixed chimerism in NOD mice has not been described either, although administration of high doses of donor BM cells and costimulatory blockade induced mixed chimerism in nonirradiated nonautoimmune mice.10,11 In the current study, high doses of donor CD8+ T cells overcame the resistance in NOD recipients and facilitate the engraftment of donor stem cells in anti-CD3–conditioned NOD recipients without irradiation. Our previous study showed that donor CD8+ T cells facilitate engraftment by eliminating residual host T cells.18
We observed that high doses of donor CD8+ T cells facilitate the engraftment of donor stem cells in nonirradiated fully major histocompatibility complex (MHC)–mismatched NOD recipients without GVHD. However, the same dose of donor CD8+ T and BM cells induced severe lethal GVHD in recipients conditioned with sublethal TBI. In the current studies, we attempted to reveal the mechanisms of GVHD prevention in anti-CD3–conditioned recipients and we found the following. (1) Compared with TBI-conditioned NOD recipients, anti-CD3–conditioned NOD recipients had markedly lower levels of serum TNF-α and markedly higher levels of serum IL-4 and IL-10. It was previously reported that proinflammatory cytokine TNF-α plays a critical role in the induction of GVHD in TBI-conditioned recipients.14,33 (2) There were only donor-type T cells in the liver of TBI-conditioned recipients but both donor- and host-type T cells in the liver of anti-CD3–conditioned recipients, and more than 70% of host-type CD4+ T cells in the liver of anti-CD3–conditioned recipients were NKT cells. It was previously reported that NKT cells were rapidly repopulated after anti-CD3–induced depletion via the proliferation of NKT precursors40 or via re-expression of TCRαβ receptors after anti-CD3 activation-induced down-regulation of TCRαβ receptors.41 Our previous studies showed that a high percentage of NKT cells presented in mice conditioned with total lymphoid irradiation (TLI), and those NKT cells prevent GVHD in TLI-conditioned BM transplant recipients via IL-4 and IL-10.34,35,42 (3) The yield of donor CD8+ T cells from the spleen of anti-CD3–conditioned recipients was significantly higher than that from TBI-conditioned recipients, but the yield of donor CD8+ T cells in the liver of anti-CD3–conditioned recipients was 10-fold lower than that of TBI-conditioned recipients. In addition, there was no lymphocyte infiltration in skin and gut tissues in chimeric NOD recipients conditioned with anti-CD3 but severe lymphocyte infiltration in those tissues of chimeric recipients conditioned with TBI. These results indicate that donor CD8+ T cells in the anti-CD3–conditioned recipients expand predominantly in the host lymphohematopoietic tissues such as spleen and LNs. In contrast, donor CD8+ T cells in TBI-conditioned recipients expand in lymphohematopoietic tissues as well as GVHD target tissues such as skin, gut, and liver. GVHD was prevented by confining donor T cells in the lymphohematopoietic tissues in previous reports.18,34,43,44
Chemokine receptors play an important role in T-cell trafficking.45,46 CCR9 and CCR10 are critical for T-cell migration to gut and skin, respectively.47,48 CCR5 and CXCR3 play a critical role in liver GVHD injury and graft rejection.43,49-51 On the other hand, the expression of chemokine receptors on T cells is regulated by both chemokines and cytokines.45,52,53 For example, CXCR3 expression is regulated by chemokine inducible protein-10 (IP-10) and cytokine IFN-γ.52,53 CCR5 and CXCR4 expression on T cells is up-regulated by IFN-γ but down-regulated by IL-4 and IL-10.54,55 Taken together, we postulate that in the TBI-conditioned recipients, donor CD8+ T cells up-regulate chemokine receptors (ie, CCR5, CCR9, CCR10, and CXCR3) in response to high levels of inflammatory chemokines and cytokines and migrate to epithelial tissues such as skin, gut, and liver to cause GVHD. In contrast, in anti-CD3–conditioned recipients, the low-level production of inflammatory cytokines and chemokines and high-level production of IL-4 and IL-10 cytokines from NKT cells prevent the up-regulation of the chemokine receptors on the donor CD8+ T cells and retain them in the lymphohematopoietic tissues. Subsequently, the injected donor CD8+ T cells may become apoptotic and anergic in the nonirradiated recipients as reported previously.15 Therefore, donor CD8+ T cells facilitate donor stem cell engraftment without GVHD in anti-CD3–conditioned recipients but induced GVHD in TBI-conditioned recipients.
In the current study, we used anti-CD3 mAb to replace TBI for conditioning of BM transplant recipients. The role of anti-CD3 conditioning is to temporarily deplete host T cells that reject donor cells. Our procedure is different from previous reports in which anti-CD3 mAb was used to prevent GVHD by depleting or blocking donor T-cell function in TBI-conditioned recipients.56,57
Multiple injections of nondepleting anti-CD3 have been reported to ameliorate diabetes in NOD mice and diabetic patients, and the therapy was associated with an increase of CD25+CD4+ regulatory T cells that suppress autoimmunity.58-60 In the current study, NOD mice conditioned with 1 injection of depleting anti-CD3 did not show any increase of CD25+CD4+ T cells during the period of T-cell recovery (data not shown). On the other hand, it is of interest to find out in future study whether the non–FcR-binding and nondepleting anti-CD3 mAb can be used to replace the depleting anti-CD3 for conditioning of BM transplant recipients in our regimen.
Veto cells in donor BM have been reported to facilitate engraftment and prevention of GVHD in BMT models,30,61 but high-dose BM alone failed to induce stable chimerism in anti-CD3–conditioned NOD recipients, indicating that the role of veto cells in our regimen is minimal.
The NOD recipients with long-term mixed chimerism showed reversal of insulitis and resistance to diabetes development despite the presence of a high percentage (about 30%) of host-type T cells in the recipients. The origin of those host-type T cells is not yet clear, but we speculate that they are de novo–developed host T cells after BMT and they are not autoreactive. We hypothesize that anti-CD3–conditioning and the injected donor CD8+ T cells eliminate the host mature T cells in the lymphohematopoietic tissues and that the donor-derived cells (such as dendritic cells) restore the negative selection function in NOD thymus and delete the autoreactive T cells so that the de novo–developed host T cells after BMT are tolerant to islet antigens. FVB/N donor superantigen-mediated deletion of NOD host T cells was found in the long-term chimeric recipients. This mechanism of restoration of self-tolerance in chimeric NOD recipients was also proposed in the previous reports.4,5
It has been reported that islet cells in diabetic mice can be regenerated to reverse overt diabetes by either self-duplication or stem cell differentiation once self-tolerance has been restored.62-64 It is of interest to test in future studies whether induction of mixed chimerism in diabetic NOD mice can promote the regeneration of islet cells and reversal of diabetes.
In conclusion, we have developed a radiation-free regimen that induces mixed chimerism in autoimmune NOD mice by taking advantage of donor CD8+ T-cell function in facilitation of donor stem cell engraftment. The separation of engraftment facilitation and GVHD mediated by donor CD8+ T cells in nonirradiated recipients has provided a new approach for induction of mixed chimerism and immune tolerance. Future efforts will be directed at finding applications of this radiation-free and GVHD-preventive regimen to the treatment of various autoimmune disorders including type 1 diabetes, as well as the induction of tolerance for islet transplantation.
Prepublished online as Blood First Edition Paper, September 16, 2004; DOI 10.1182/blood-2004-06-2411.
Supported by The Leslie and Susan Gonda (Goldschmied) Foundation, The National Institute of Health (National Institute of Allergy and Infectious Diseases [NIAID] 42288), and The Juvenile Diabetes Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lucy Brown and Claudia Spalla at City of Hope (COH) Flow Cytometry Facility and Sofia Loera at COH Anatomic Pathology Laboratory and Clive Wasserfall and Fletcher Schwartzof the University of Florida for their excellent technical assistance. We are grateful to Dr Mitchell Kronenberg at La Jolla Institute for at COH Graduate School, Megan Sykes at Harvard University, and Samuel Strober at Stanford University for their critical review of providing us with CD1d-αGalCer-tetramer and to Drs Tom Lebon our manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal