Abstract
CD4+CD25+ regulatory T (Treg) cells are potent modulators of alloimmune responses. In murine models of allogeneic bone marrow transplantation, adoptive transfer of donor CD4+CD25+ Treg cells protects recipient mice from lethal acute graft-versus-host disease (aGVHD) induced by donor CD4+CD25- T cells. Here we examined the differential effect of CD62L+ and CD62L- subsets of CD4+CD25+ Treg cells on aGVHD-related mortality. Both subpopulations showed the characteristic features of CD4+CD25+ Treg cells in vitro and did not induce aGVHD in vivo. However, in cotransfer with donor CD4+CD25- T cells, only the CD62L+ subset of CD4+CD25+ Treg cells prevented severe tissue damage to the colon and protected recipients from lethal aGVHD. Early after transplantation, a higher number of donor-type Treg cells accumulated in host mesenteric lymph node (LN) and spleen when CD4+CD25+CD62L+ Treg cells were transferred compared with the CD62L- subset. Subsequently, CD4+CD25+CD62L+ Treg cells showed a significantly higher capacity than their CD62L- counterpart to inhibit the expansion of donor CD4+CD25- T cells. The ability of Treg cells to efficiently enter the priming sites of pathogenic allo-reactive T cells appears to be a prerequisite for their protective function in aGVHD.
Introduction
CD4+CD25+ regulatory T (Treg) cells are potent modulators of immune responses. We and others have demonstrated that donor-derived CD4+CD25+ Treg cells could suppress lethal acute graft-versus-host disease (aGVHD) in murine models of allogeneic bone marrow transplantation.1-3 In these models, cotransplantation of Treg cells with conventional donor T cells controlled the expansion of alloaggressive T cells in recipient animals, thereby interfering with one of the major events in the initiation phase of aGVHD.4 Importantly, donor Treg cells did not cause generalized immune paralysis, since the beneficial graft-versus-leukemia/lymphoma (GVL) effect of donor T cells was maintained.4-6 Modulating alloimmune responses after bone marrow or hematopoietic stem cell transplantation with adoptively transferred donor CD4+CD25+ Treg cells thus appears as a promising strategy for the prevention or therapy of aGVHD in humans.
CD62L (L-selectin) is an important T-cell homing receptor as well as a marker for T-cell development. Naive T cells are CD62L+ and interaction of CD62L with its ligands, a group of molecules collectively referred to as peripheral node addressin (PNAd), is crucial for T-cell entry into lymph nodes (LNs) via high endothelial venules.7 Expression of CD62L is rapidly lost following T-cell receptor engagement, and CD62L- T cells are thought to be “antigen experienced.” There are 2 recent publications demonstrating that CD62L- donor T cells did not cause GVHD.8,9 While CD4+CD62L- T cells contained a higher fraction of CD4+CD25+ Treg cells, their inability to induce GVHD was maintained after Treg cell depletion.8 Nevertheless, these findings called for the reciprocal analysis of the GVHD-regulating capacity of CD62L+ and CD62L- subsets of CD4+CD25+ Treg cells.
Comparing the effects CD4+CD25+CD62L+ and CD62L- Treg cell subpopulations in aGVHD was suggested by a second line of investigations. Both subsets had been shown to be equally anergic and suppressive upon polyclonal stimulation in vitro.10-12 Interestingly, we found that in an adoptive transfer model of diabetes into nonobese diabetic–severe combined immunodeficient (NOD-scid) mice, only the CD62L+ but not the CD62L- subset of CD4+CD25+ Treg cells caused a significant delay of disease onset.12 In contrast, another group reported recently that both CD62L+ and CD62L- Treg cell subsets were protective in an adoptive transfer model of colitis,13 suggesting that the inconsistency between in vitro and in vivo experiments in NOD mice may have been related to peculiarities of this mouse strain, which spontaneously develops autoimmune diabetes. In the current study, we used the aGVHD model as an additional in vivo assay for suppressor function in a nonautoimmune disease-prone strain.
Using a mouse model for lethal aGVHD induced by major histocompatibility complex (MHC)–mismatched CD4+CD25- T cells,1 we found that only the CD62L+ subpopulation of CD4+CD25+ T cells protected recipients against GVHD-related severe tissue damage and death. As reported before, both CD62L+ and CD62L- subsets displayed the characteristic features of CD4+CD25+ Treg cells in vitro. However, CD4+CD25+CD62L+ Treg cells showed a significantly higher capacity to home to secondary lymphoid organs in vivo and subsequently inhibit the expansion of pathogenic CD4+CD25- donor T cells. Our results suggest that the ability of Treg cells to efficiently enter the priming sites of pathogenic alloreactive T cells is a prerequisite for their protective function in aGVHD.
Materials and methods
Mice
C57BL/6 (H-2KbThy1.2Ly5.1) and BALB/c (H-2Kd) mice were obtained from the breeding facility of the Department of Comparative Medicine, Stanford University. C57BL/6.Thy1.1 and C57BL/6.Ly5.2 congenic mice were provided by the laboratory of Dr Irving Weissman, Stanford University. Only male mice were used for experiments. Donors were between 6 and 12 weeks of age; recipients were at least 8 weeks old. Care of all experimental animals was in accordance with institutional guidelines.
Antibodies and flow cytometry
The following reagents were used for flow cytometric analysis: unconjugated anti-CD16/32 (2.4G2), anti-CD25 allophycocyanin (APC) (PC61), anti-CD62L fluorescein isothiocyanate (FITC, Mel-14), anti-CD69 phycoerythrin (PE, H1.2F3), anti-CD44 PE (IM7), anti-CD45RB PE (16A), and anti–H-2Kb FITC (AF6-88.5); streptavidin PE (SA/PE) were purchased from BD Pharmingen (San Diego, CA). Anti-CD4 Cy7/APC (RM4-5) was from Caltag (South San Francisco, CA). The anti-glucocorticoid induced TNF receptor (GITR) clone 3H12 was kindly provided by Dr Shimon Sakaguchi, Kyoto University. The antibody was purified and biotinylated according to standard protocols. Biotinylated anti-Thy1.1 and anti-Ly5.2 were kindly provided by Dr Weissman's laboratory. Stainings were performed in the presence of purified anti-CD16/32 at saturation to block unspecific staining. Propidium iodide (Sigma, St Louis, MO) was added prior to analysis to exclude dead cells. All analytical flow cytometry was done on a modified dual laser LSRScan (BD Immunocytometry Systems, San Diego, CA) in the Shared FACS Facility, Center for Molecular and Genetic Medicine at Stanford using FlowJo software (TreeStar, Ashland, OR) for data analysis.
Cell isolation and sorting
Single cell-suspensions from spleens were enriched for CD25+ cells after sequential staining with anti-CD25 PE (BD PharMingen) and anti-PE magnetic beads using the autoMACS system (Miltenyi Biotec, Auburn, CA). The negative fraction from this separation was enriched for CD4+ cells with anti-CD4 magnetic beads. CD25+ and CD25-CD4+ cells were then stained with anti-CD4 APC and anti-CD62L FITC and sorted on a FACS Vantage (Becton Dickinson, Mountain View, CA). T-cell–depleted bone marrow (TCD BM) was obtained through negative depletion using anti-Thy1.2 magnetic beads (Miltenyi Biotech). Thy1.2-depleted splenocytes were used as allogenic stimulator cells.
GVHD model
aGVHD was induced as described previously.1 In brief, BALB/c hosts were lethally irradiated (800 cGy) and injected intravenously within 24 hours with 2 × 106 TCD BM cells for reconstitution plus any additional cells. For survival studies, mice were kept on antibiotic water (neomycin/polymyxin) for the first 28 days. Survival and appearance were monitored daily. For histology, mice were killed 5 days after cell transfer. Hematoxylin/eosin (H/E) staining of paraffin-embedded tissue sections was performed according to standard protocols. Image acquisition was done using an Eclipse E1000 microscope (Nikon, Melville, NY) with SPOT RT camera and acquisition software (Diagnostic Instruments, Sterling Heights, MI).
Cell distribution studies
Cell preparation and aGVHD induction were performed as described. For day-2 biodistribution studies, the cell population of interest was labeled with 111In-oxine.14 Briefly, sorted CD4+CD25+CD62L+ and CD62L- T cells were incubated in 150 to 200 μL (0.15 to 0.20 mCi of activity; 5.55-7.4 MBq) of stock 111In-oxine (Amersham Health, San Jose, CA) for 30 minutes at room temperature. The cells were then spun, washed once with phosphate-buffered saline (PBS), resuspended in medium, and, after admixing unlabeled CD4+CD25- T cells and TCD BM, injected into irradiated hosts. Recipients were killed 48 hours later, and the various organs were dissected and their weight was recorded. Organ samples were analyzed in a scintillation well counter along with 3 samples of standard activity (1/100 of injected dose) at 2 energy windows, 100 to 200 and 210 to 350 keV. Results were expressed percentage of injected dose (ID) per gram (g) of tissue (% ID/g). For day-5 analysis, Thy1.1 or Ly5.2 congenic C57BL/6 mice were used as donors for the CD4+CD25- T cells. Recipients were killed 5 days after aGVHD induction. Cell suspensions from mesenteric LNs and spleen were prepared using a syringe plunger. Mononuclear cells from the liver were isolated by gradient centrifugation as described by Eberl and MacDonald.15 Viable cells were counted, stained with appropriate antibodies, and analyzed by FACS.
Mixed lymphocyte reactions and polyclonal stimulation assays
Cultures were set up in 96-well round-bottom plates (BD Biosciences, Franklin Lakes, NJ) in a total volume of 200 μL. Cells were cultured in RPMI-C that is RPMI 1640 (Bio Whittaker, Walkersville, MD) supplemented with 10% heat-inactivated fetal bovine serum, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1% nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin + 100 μg/mL streptomycin, 2 mM l-glutamine (all Gibco BRL, Gaithersburg, MD), and 50 μM 2-mercaptoethanol (Sigma). CD4+CD25- responder cells and irradiated (3000 cGy) allogenic stimulator cells (100 000 cells each) were mixed with variable numbers of CD4+CD25+ T cells to obtain the ratios indicated. Proliferation was assessed after 5 days by pulsing the cells with 1 μCi/well (0.037 MBq) 3H-thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) for the last 16 hours. Cells were harvested onto filter membranes using a Wallac harvester (PerkinElmer Life Sciences, Gaithersburg, MD), and the amount of incorporated 3H-thymidine was measured with a Wallac Betaplate counter (PerkinElmer Life Sciences).
IL-2 ELISA
Cells (25 000) were incubated in 200 μL RPMI-C with phorbol myristate acetate (PMA, 50 ng/mL; Sigma) plus ionomycin (1 μM; Calbiochem, La Jolla, CA) in 96-well flat-bottom plates. Supernatants were harvested after 24 hours and analyzed for interleukin-2 (IL-2) by enzyme-linked immunosorbent assay (ELISA) as described.16 Values presented are the mean and standard deviation of triplicate cultures.
Real-time quantitative PCR
Total mRNA was isolated from frozen cell pellets using the RNeasy MiniKit (Qiagen, Valencia, CA) and after digestion of genomic DNA (DNA-free; Ambion, Austin, TX) reverse-transcribed with TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). Quantitative real-time polymerase chain reaction (PCR) was then performed on a MX4000 (Stratagene, La Jolla, CA) using the Brilliant SYBR Green QPCR Master Mix (Stratagene) and the following primers: FoxP3 forward, CCGCAAGCTAAAAGCCAGG; FoxP3 reverse, CTTTGCCTTCGTGCCCACT; β-actin forward, GACGGCCAAGTCATCACTATTG; and β-actin reverse, AGGAAGGCTGGAAAAGAGCC.
Statistical analysis
Statistical analysis was performed using Prism (GraphPad Software, San Diego, CA). Differences in animal survival were analyzed by log-rank test. Cell numbers recovered from various organs were compared using the Mann-Whitney test.
Results
CD4+CD25+CD62L- T cells do not protect from lethal aGVHD
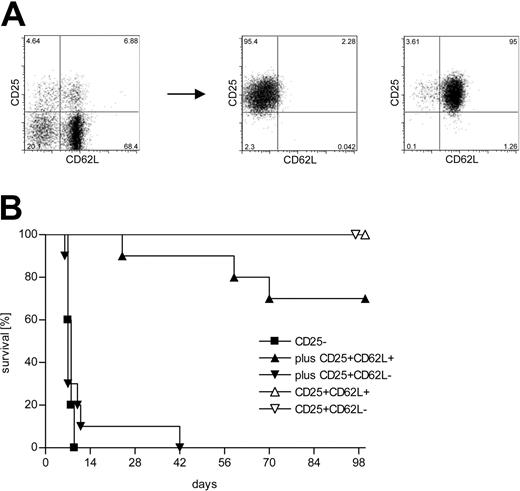
Lethal aGVHD can be induced by adoptive transfer of a limited number of CD4+CD25- C57BL/6 (H-2b) T cells into lethally irradiated BALB/c (H-2d) hosts. Priming and massive expansion of alloreactive donor T cells together with severe inflammation of the gut leads to clinically apparent severe diarrhea and death of the animals within 1 to 2 weeks. Using this model, we have previously shown that cotransfer of donor-derived CD4+CD25+ Treg cells at a 1:1 ratio protects against lethal disease.1 About 40% of the CD4+CD25+ T cells in the spleen of an 8-week-old C57BL/6 mouse are CD62L-. We purified CD4+CD25+CD62L+ and CD62L- T cells from C57BL/6 donors to more than 95% purity (Figure 1A) and used them in our aGVHD model. Figure 1B demonstrates that the cotransfer of CD4+CD25+CD62L- T cells did not lead to a significant survival benefit compared with transfer of CD4+CD25- T cells alone (P = .62). The mice in these 2 experimental groups rapidly developed severe aGVHD and all mice had succumbed to the disease by day 42 after transplantation. In contrast, about 70% of the mice that received CD4+CD25+CD62L+ T cells together with CD4+CD25- T cells survived for more than 100 days (P < .0001). Survivors appeared normal at 100 days and showed no skin abnormalities, hunched back, or diarrhea. None of the animals that received either CD4+CD25+CD62L+ or CD4+CD25+CD62L- T cells alone developed any clinical signs of aGVHD and all survived for at least 100 days.
Only the CD62L+ subset of CD4+CD25+ Treg cells protects from lethal aGVHD in vivo. (A) CD4+ splenocytes from C57BL/6 donors were sorted into CD4+CD25- (not shown), CD4+CD25+CD62L+, and CD4+CD25+CD62L- subpopulations. (B) Lethally irradiated BALB/c recipients received 2 × 106 TCD BM cells from C57BL/6 mice for reconstitution plus 500 000 C57BL/6-derived CD4+CD25- T cells either alone (▪; n = 10) or together with 500 000 CD4+CD25+CD62L+ (▴; n = 10) or CD4+CD25+CD62L- (▾; n = 10) Treg cells. Additional groups were injected with TCD BM and 500 000 CD4+CD25+CD62L+ (▵; n = 5) or CD4+CD25+CD62L- (▵; n = 3) Treg cells only. Data were pooled from 2 independent experiments.
Only the CD62L+ subset of CD4+CD25+ Treg cells protects from lethal aGVHD in vivo. (A) CD4+ splenocytes from C57BL/6 donors were sorted into CD4+CD25- (not shown), CD4+CD25+CD62L+, and CD4+CD25+CD62L- subpopulations. (B) Lethally irradiated BALB/c recipients received 2 × 106 TCD BM cells from C57BL/6 mice for reconstitution plus 500 000 C57BL/6-derived CD4+CD25- T cells either alone (▪; n = 10) or together with 500 000 CD4+CD25+CD62L+ (▴; n = 10) or CD4+CD25+CD62L- (▾; n = 10) Treg cells. Additional groups were injected with TCD BM and 500 000 CD4+CD25+CD62L+ (▵; n = 5) or CD4+CD25+CD62L- (▵; n = 3) Treg cells only. Data were pooled from 2 independent experiments.
CD62L+ and CD62L- subsets of CD4+CD25+ T cells differ with regard to the expression of various memory/activation markers
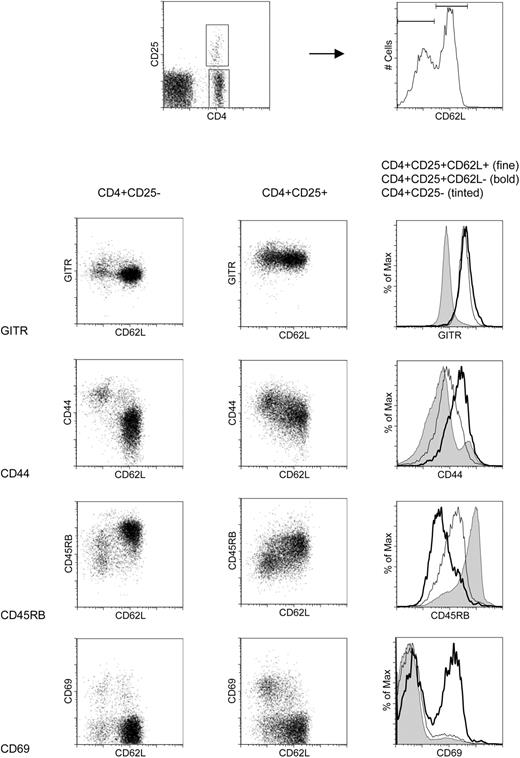
We next analyzed CD4+CD25+CD62L+ and CD62L- splenocytes from 8-week-old C57BL/6 mice for the expression of other memory/activation markers using 4-color flow cytometry (Figure 2). Both subpopulations had uniformly high surface levels of GITR, a marker that has been described to be preferentially expressed on CD4+CD25+ Treg cells and to modulate Treg cell function.17,18 CD4+CD25+ Treg cells are known to have a memory phenotype as defined by expression of CD45RB and CD44.10 Interestingly, while the CD62L- subset was truly CD45RBlow and CD44high, intermediate expression levels of CD45RB and CD44 were observed for the CD62L+ subset. Furthermore, about 50% of CD4+CD25+CD62L- T cells were activation marker CD69+ compared with only 15% of the CD4+CD25+CD62L+ T cells, suggesting that the CD4+CD25+CD62L- subset might contain a considerable fraction of recently activated conventional CD4+ T cells.
CD4+CD25+CD62L+ and CD4+CD25+CD62L- T cells express comparable levels of GITR on their surface but differ in the expression of other markers. C57BL/6 splenocytes were stained with anti-CD4 Cy7APC, anti-CD25 APC, anti-CD62L FITC, and PE-labeled antibody against the marker of interest and analyzed by flow cytometry. Two-dimensional dot plots of CD62L versus GITR, CD44, CD45RB, or CD69 are shown for CD4+CD25- T cells in the left and CD4+CD25+ T cells in the middle column. The histograms in the right column are overlays of the respective markers for CD4+CD25- T cells (shaded), CD4+CD25+CD62L- (bold), and CD4+CD25+CD62L+ Treg cells (fine). The gates for CD4+CD25- and CD4+CD25+ cells as well as the CD62L+ and CD62L- subsets are shown in the two top FACS plots.
CD4+CD25+CD62L+ and CD4+CD25+CD62L- T cells express comparable levels of GITR on their surface but differ in the expression of other markers. C57BL/6 splenocytes were stained with anti-CD4 Cy7APC, anti-CD25 APC, anti-CD62L FITC, and PE-labeled antibody against the marker of interest and analyzed by flow cytometry. Two-dimensional dot plots of CD62L versus GITR, CD44, CD45RB, or CD69 are shown for CD4+CD25- T cells in the left and CD4+CD25+ T cells in the middle column. The histograms in the right column are overlays of the respective markers for CD4+CD25- T cells (shaded), CD4+CD25+CD62L- (bold), and CD4+CD25+CD62L+ Treg cells (fine). The gates for CD4+CD25- and CD4+CD25+ cells as well as the CD62L+ and CD62L- subsets are shown in the two top FACS plots.
Both CD62L+ and CD62L- subsets of CD4+CD25+ T cells show Treg cell characteristics in vitro
It has been reported that both CD62L+ and CD62L- subsets of CD4+CD25+ T cells can suppress the proliferation of CD4+CD25- T cells upon polyclonal stimulation in vitro.10,11 Figure 3A demonstrates that this holds true for allogeneic stimulation. Both CD4+CD25+CD62L+ and CD4+CD25+CD62L- T cells from C57BL/6 mice failed to proliferate in response to BALB/c stimulator cells but suppressed the proliferation of cocultured C57BL/6 CD4+CD25- T cells in a dose-dependent manner with similar efficiency. The anergic phenotype of both subsets was further tested by stimulation with PMA/ionomycin. In contrast to CD4+CD25- T cells, neither CD4+CD25+CD62L+ nor CD4+CD25+CD62L- T cells produced significant amounts of IL-2 when exposed to this strong stimulus (Figure 3B). Expression of the transcription factor Foxp3 has recently been linked to a Treg cell phenotype.19,20 We analyzed FoxP3 mRNA levels by quantitative real-time PCR (Figure 3C). No difference in FoxP3 expression could be detected between the 2 CD4+CD25+ T-cell subsets, while both had about 25-fold higher FoxP3 levels than CD4+CD25- T cells. Regardless of the differences in expression of additional cell surface markers, the data presented in Figure 3 demonstrate that both CD4+CD25+CD62L+ and CD4+CD25+CD62L- T cells share key characteristics of Treg cells. In fact, the quantitative similarity with respect to their suppressive capacity, anergic phenotype, and FoxP3 expression level is evidence against a major contamination of the CD4+CD25+CD62L- subset with recently activated conventional T cells.
Both CD4+CD25+CD62L+ and CD4+CD25+CD62L- T cells show Treg cell characteristics in vitro. (A) Alloresponse of C57BL/6-derived CD4+CD25- T cells and CD4+CD25+CD62L+ and CD62L- T cells toward BALB/c APC in vitro. Cultures were set up with 100 000 BALB/c-derived APC and 100 000 sorted CD4+CD25- T cells from C57BL/6 mice plus variable numbers of C57BL/6-derived CD4+CD25+CD62L+ (▨) or CD62L- T cells (▪) to obtain the indicated ratios. CD4+CD25+CD62L+ and CD62L- T cells were also stimulated alone. Proliferation was assessed by labeling the cultures with 3H-thymidine for the final 16 hours of the 5-day incubation period. Data represent mean + SD of triplicate cultures. Shown is 1 of 5 experiments with similar results. (B) Sorted CD4+CD25+CD62L- (▪), CD4+CD25+CD62L+ (▨), and CD4+CD25- T cells (□; 25 000) were stimulated for 24 hours with 50 ng/mL PMA and 1 μM ionomycin. IL-2 in the supernatant was determined by ELISA. The results represent mean + SD of triplicate cultures. (C) cDNA was prepared from cell populations sorted as in panel B and analyzed for expression of FoxP3 by real-time quantitative PCR using β-actin as normalizing gene.
Both CD4+CD25+CD62L+ and CD4+CD25+CD62L- T cells show Treg cell characteristics in vitro. (A) Alloresponse of C57BL/6-derived CD4+CD25- T cells and CD4+CD25+CD62L+ and CD62L- T cells toward BALB/c APC in vitro. Cultures were set up with 100 000 BALB/c-derived APC and 100 000 sorted CD4+CD25- T cells from C57BL/6 mice plus variable numbers of C57BL/6-derived CD4+CD25+CD62L+ (▨) or CD62L- T cells (▪) to obtain the indicated ratios. CD4+CD25+CD62L+ and CD62L- T cells were also stimulated alone. Proliferation was assessed by labeling the cultures with 3H-thymidine for the final 16 hours of the 5-day incubation period. Data represent mean + SD of triplicate cultures. Shown is 1 of 5 experiments with similar results. (B) Sorted CD4+CD25+CD62L- (▪), CD4+CD25+CD62L+ (▨), and CD4+CD25- T cells (□; 25 000) were stimulated for 24 hours with 50 ng/mL PMA and 1 μM ionomycin. IL-2 in the supernatant was determined by ELISA. The results represent mean + SD of triplicate cultures. (C) cDNA was prepared from cell populations sorted as in panel B and analyzed for expression of FoxP3 by real-time quantitative PCR using β-actin as normalizing gene.
CD4+CD25+CD62L+ T cells home more efficiently to secondary lymphoid organs than CD4+CD25+CD62L- T cells
We hypothesized that differential trafficking as a consequence of the presence or absence of CD62L combined with differential expression of chemokine receptors and other homing molecules might explain the functional differences between CD4+CD25+CD62L+ and CD62L- Treg cells in vivo. We previously reported for NOD mice12 and have confirmed for C57BL/6 mice (J.E., data not shown, June 2003) that CD4+CD25+CD62L+ and CD4+CD25+CD62L- splenocytes differ significantly in their expression of chemokine receptors. To directly address the homing hypothesis, we labeled CD4+CD25+CD62L+ and CD62L- T cells, respectively, with 111In-oxine and injected them together with unlabeled CD4+CD25- T cells and TCD BM into irradiated hosts. The biodistribution of labeled cells was analyzed 48 hours later by measuring radioactivity in various organs. To demonstrate organ-specific enrichment, we normalized the recovered dose over organ weight. Figure 4 demonstrates that the CD62L+ Treg cell subset homed significantly better to the mesenteric LN than the CD62L- subset (factor, 2.6; P = .0003). A similar trend was seen for migration to peripheral LNs (inguinal + axillary LNs: factor, 2.2; P = .054) and to the spleen (factor, 1.6, P = .19). No differences were observed in accumulation of signal in liver (P = .46), small bowel (P = .78), large bowel (P = .87), or other organs (data not shown).
CD4+CD25+CD62L+ T cells home significantly better to secondary lymphoid tissues than CD4+CD25+CD62L- T cells. Sorted CD4+CD25+CD62L+ (▨) and CD4+CD25+CD62L- T cells (▪) were labeled with 111In before cotransfer with unlabeled CD4+CD25- T cells and TCD BM into irradiated hosts. Mice were killed 48 hours later and radioactivity in the various organs was measured. (A) Global comparison of aGVHD target organs (liver, small bowel, large bowel) and primary lymphoid organs (spleen, peripheral LN [PLN], mesenteric LN [MLN]). Results are expressed as fraction of injected radioactive dose divided by organ weight in grams (% ID/g) representing organ-specific enrichment. Mean + SD is given. (B) Results from individual mice are shown for mesenteric LN, spleen, and liver. The horizontal line represents the group median. P values are provided above the figure. Data were pooled from 3 experiments (CD4+CD25+CD62L+ mice, n = 7; CD4+CD25+CD62L- mice, n = 8).
CD4+CD25+CD62L+ T cells home significantly better to secondary lymphoid tissues than CD4+CD25+CD62L- T cells. Sorted CD4+CD25+CD62L+ (▨) and CD4+CD25+CD62L- T cells (▪) were labeled with 111In before cotransfer with unlabeled CD4+CD25- T cells and TCD BM into irradiated hosts. Mice were killed 48 hours later and radioactivity in the various organs was measured. (A) Global comparison of aGVHD target organs (liver, small bowel, large bowel) and primary lymphoid organs (spleen, peripheral LN [PLN], mesenteric LN [MLN]). Results are expressed as fraction of injected radioactive dose divided by organ weight in grams (% ID/g) representing organ-specific enrichment. Mean + SD is given. (B) Results from individual mice are shown for mesenteric LN, spleen, and liver. The horizontal line represents the group median. P values are provided above the figure. Data were pooled from 3 experiments (CD4+CD25+CD62L+ mice, n = 7; CD4+CD25+CD62L- mice, n = 8).
CD4+CD25+CD62L+ T cells inhibit the expansion of alloreactive CD4+CD25- T cells in vivo more efficiently than CD4+CD25+CD62L- T cells and protect against GVHD-related tissue damage to the large intestine
To better understand subsequent events in the interaction between cotransferred CD4+CD25+ and CD4+CD25- T cells, we used congenic C57BL/6 mice (Thy1.1 or Ly5.2) as donors of the CD4+CD25- T cells. It was thus possible to identify the progeny of these cells as H-2Kb+CD4+[congenic marker]+ and the progeny of the cotransferred CD4+CD25+ subsets as H-2Kb+CD4+[congenic marker]-. CD4+CD25- T cells (500 000) were injected together with TCD BM into irradiated BALB/c hosts either alone (control hosts) or together with 500 000 CD4+CD25+CD62L+ or CD4+CD25+CD62L- T cells (experimental hosts). Recipients were killed on day 5 (shortly before the first control hosts were expected to die). Single-cell suspensions from mesenteric LN and spleen and mononuclear cells from the liver were prepared. Viable cells were counted and analyzed by FACS as described. Since there was considerable variation in donor CD4+ T-cell yield in the 4 independent experiments performed, we normalized the data by calculating the ratio of T-cell numbers recovered from individual experimental hosts over the average number of donor CD4+CD25- T cells recovered from control hosts within a given experiment. As demonstrated in Figure 5A, we could recover 2.3 times more CD4+CD25+CD62L+ than CD4+CD25+CD62L- T cells from mesenteric LN (P = .0003) and 2.7 times more from the spleen (P = .004). Recovery from the liver was similar (P = .46). CD62L expression levels on all recovered Treg cells were low (data not shown). For CD4+CD25- T-cell progeny, the normalized cell count represents the expansion of CD4+CD25- T cells in the presence of Treg cells relative to their expansion when transferred alone. Figure 5B shows that both CD4+CD25+CD62L+ and CD62L- Treg cell subsets inhibited the proliferation of CD4+CD25- T cells (P < .001 for all groups and organs), although to a different degree. The expansion of donor CD4+CD25- T cells in the mesenteric LN was more strongly inhibited in mice that had received additional CD4+CD25+CD62L+ T cells than in recipients of the CD62L- subset (median, 0.36 vs 0.65; P = .017). Similar relative reductions were seen in the spleen (0.17 vs 0.33; P = .033) and liver (0.33 vs 0.67; P = .002). In Figure 5C, we calculated the fraction of donor CD4+ T cells that was derived from CD4+CD25+ T cells. This is an important parameter as it has been shown both in vitro and in vivo1 that the protective effect of CD4+CD25+ Treg cells is dose dependent. CD4+CD25+ Treg cells made up a considerably larger fraction of all donor CD4+ T cells after transfer of the CD62L+ subset than after transfer of CD62L- cells (median, 0.20 vs 0.05 in MLN, P = .0003; 0.21 vs 0.08 in spleen, P = .0003; 0.13 vs 0.05 in liver, P = .001).
CD4+CD25+CD62L- T cells are quantitatively less efficient suppressors in vivo. Lethally irradiated BALB/c mice received TCD BM and CD4+CD25- T cells from congenic C57BL/5 without (control hosts) or together with (experimental hosts) CD4+CD25+CD62L+ or CD4+CD25+CD62L- Treg cells from wild-type (WT) C57BL/6 donors. All mice were killed 5 days after transfer. Single cell suspensions were prepared from mesenteric LN (MLN), spleen, and liver of individual mice. Viable cells were counted, stained with appropriate antibodies, and analyzed by flow cytometry. The progeny of CD4+CD25- and CD4+CD25+ T cells was identified as CD4+H-2Kb+[congenic marker]+/-. Results for individual mice are shown. The horizontal line represents the group median. P values are given above the figures. Data were pooled from 4 experiments with 9 to 11 mice per group. (A) Recovery of CD4+CD25+ T cells after transfer of the CD62L+ or CD62L- subset. (B) Expansion of CD4+CD25- T cells in mesenteric LN, spleen, or liver after cotransfer of CD4+CD25+CD62L+ or CD4+CD25+CD62L- Treg cells. (C) Fraction of CD4+CD25+ T-cell progeny among all donor CD4+ T cells in individual mice.
CD4+CD25+CD62L- T cells are quantitatively less efficient suppressors in vivo. Lethally irradiated BALB/c mice received TCD BM and CD4+CD25- T cells from congenic C57BL/5 without (control hosts) or together with (experimental hosts) CD4+CD25+CD62L+ or CD4+CD25+CD62L- Treg cells from wild-type (WT) C57BL/6 donors. All mice were killed 5 days after transfer. Single cell suspensions were prepared from mesenteric LN (MLN), spleen, and liver of individual mice. Viable cells were counted, stained with appropriate antibodies, and analyzed by flow cytometry. The progeny of CD4+CD25- and CD4+CD25+ T cells was identified as CD4+H-2Kb+[congenic marker]+/-. Results for individual mice are shown. The horizontal line represents the group median. P values are given above the figures. Data were pooled from 4 experiments with 9 to 11 mice per group. (A) Recovery of CD4+CD25+ T cells after transfer of the CD62L+ or CD62L- subset. (B) Expansion of CD4+CD25- T cells in mesenteric LN, spleen, or liver after cotransfer of CD4+CD25+CD62L+ or CD4+CD25+CD62L- Treg cells. (C) Fraction of CD4+CD25+ T-cell progeny among all donor CD4+ T cells in individual mice.
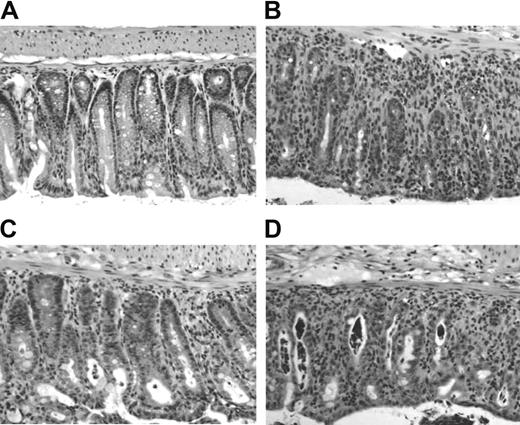
Finally, we looked at histologic changes in target tissues of aGVHD. We have published previously that in this model of aGVHD the most severe tissue damage occurs in skin and colon with minimal changes in liver and small intestine by day 40.21 In this study, smaller donor T-cell numbers were transferred resulting in a more protracted disease course. Here, we killed recipient mice after 5 days. Samples of liver, small and large intestine were fixed, processed, and stained with hematoxylin/eosin. Although we were able to isolate and analyze single cell suspensions from the liver on day 5 after cell transfer (Figure 5), there was no histologic evidence of GVHD in the liver at that time (data not shown). Very mild histologic changes were observed in the small intestine of all mice (data not shown). In contrast, clear signs of GVHD could be detected in the large intestine of CD4+CD25- control hosts, confirming our previous report and suggesting that the colon is the primary target organ in this model system. Figure 6 shows a moderate to severe degree of mononuclear cell infiltration with disruption of the mucosal crypt architecture and apoptosis of enterocytes. Interestingly, recipients of CD4+CD25- T cells and CD4+CD25+CD62L- Treg cells (Figure 6D) were histologically indistinguishable from CD4+CD25- control hosts (Figure 6B), while those that received additional CD4+CD25+CD62L+ Treg cells (Figure 6C) showed only mild histologic changes and resembled more the TCD BM control (Figure 6A).
Minimal histologic damage in the large intestine 5 days after cotransfer of CD4+CD25+CD62L+ Treg cells. Lethally irradiated BALB/c hosts received TCD BM or TCD BM plus 500 000 CD4+CD25- T cells alone or together with 500 000 CD4+CD25+CD62L+ or CD4+CD25+CD62L- T cells. Animals (n = 3 each group) were killed 5 days later, and a piece of large bowel was processed for standard H/E histology. Representative sections are shown at 1:40 magnification for recipients of (A) TCD BM alone, (B) CD4+CD25- T cells, (C) CD4+CD25- plus CD4+CD25+CD62L+ T cells, and (D) CD4+CD25- plus CD4+CD25+CD62L- T cells.
Minimal histologic damage in the large intestine 5 days after cotransfer of CD4+CD25+CD62L+ Treg cells. Lethally irradiated BALB/c hosts received TCD BM or TCD BM plus 500 000 CD4+CD25- T cells alone or together with 500 000 CD4+CD25+CD62L+ or CD4+CD25+CD62L- T cells. Animals (n = 3 each group) were killed 5 days later, and a piece of large bowel was processed for standard H/E histology. Representative sections are shown at 1:40 magnification for recipients of (A) TCD BM alone, (B) CD4+CD25- T cells, (C) CD4+CD25- plus CD4+CD25+CD62L+ T cells, and (D) CD4+CD25- plus CD4+CD25+CD62L- T cells.
Discussion
We have previously shown that donor-type CD4+CD25+ T cells were able to protect mice from lethal aGVHD induced by CD4+CD25- T cells across a complete MHC mismatch barrier. We show now that, using CD62L expression as a marker, CD4+CD25+ T cells can be divided into 2 subpopulations with distinct functional differences in vivo. Although both CD4+CD25+CD62L+ and CD4+CD25+CD62L- subsets have Treg cell characteristics when analyzed in vitro, only the CD62L+ subpopulation protects recipients from death in a mouse model of aGVHD.
It has been recognized that T-cell trafficking plays a central role in the pathogenesis of GVHD.22 Both induction of murine GVHD8,9 and, as demonstrated in this paper, protection from GVHD-induced lethality are exerted by T-cell populations that express CD62L on their surface. One plausible interpretation of these findings is that the priming of alloreactive conventional CD4+ T cells as well as the inhibition of their expansion by donor-derived Treg cells occurs in a location that requires the interaction of CD62L on the T-cell surface with PNAd or alternative endothelial ligands23 for efficient entry. This is supported by reports that in 2 different mouse models blockade of CD62L (and CD49d) redirected donor T cells in vivo and ameliorated GVHD.24,25 It appears that in our aGVHD model, the mesenteric LN is this crucial “CD62L-accessible” priming site. After transfer into irradiated hosts, disease-inducing CD4+CD25-CD62L+ T cells accumulate rapidly in all host LNs. However, it is in the mesenteric LN and not in other peripheral LNs where they rapidly expand (J.E., unpublished data, December 2002). Importantly, we demonstrate in this paper that CD4+CD25+CD62L+ Treg cells home significantly better to secondary lymphoid organs than their CD62L- counterparts. This allows them to more efficiently inhibit the proliferation of alloreactive CD4+CD25- T cells in the mesenteric LN and protect the host from overwhelming aGVHD and death. A trafficking pattern that more (or less) overlaps with that of the pathogenic CD4+CD25-CD62L+ T cells can thus explain the differential ability of CD4+CD25+CD62L+ and CD62L- T cells to protect from lethal aGVHD.
The lack of protection by the CD4+CD25+CD62L- Treg cell subset in terms of survival is striking. However, the day-5 data clearly demonstrate that this subset does have some inhibitory effect on the expansion on CD4+CD25- T cells in vivo. Reciprocally, the fact that 70% of the mice that received CD4+CD25- T cells together with CD4+CD25+CD62L+ Treg cells survived more than 100 days does not mean that these mice were completely protected from GVHD development. We have previously published1 that some recipients of CD4+CD25- plus total CD4+CD25+ T cells showed clinical signs of GVHD at 4 to 5 weeks after transfer, but recovered thereafter and survived long term. Furthermore, there was histologic evidence of mild GVHD affecting the skin and gut at 7 weeks after cotransfer of CD4+CD25- and CD4+CD25+ T cells, but not at 100 days. Our interpretation of these findings is that CD4+CD25+ T cells do not completely prevent the activation of allospecific conventional T cells and that GVHD develops to some degree. This view is further supported by the demonstration that CD4+CD25+ Treg cells prevented death from acute GVHD but still allowed a protective GVL response to occur in 2 mouse tumor models.4 The data presented in this manuscript are consistent with our previously published results, in that some animals who had received CD4+CD25+CD62L+ Treg cells together with CD4+CD25- T cells died between 4 and 10 weeks after transplantation. The long-term survivors in this group had no clinical signs of GVHD at the end of the experiment. They looked normal without skin changes, hunched back, or diarrhea. Thus, they had the appearance of mice that had received unfractionated CD4+CD25+ Treg cells as described in our previous report.1
In conventional CD4+CD25- T cells, expression of CD62L distinguishes between naive and effector memory T cells.26 The application of this paradigm to CD4+CD25+ T cells is problematic as very little is known about antigen specificity and antigen experience of these cells. Recently developed transgenic mouse systems allow the analysis of CD4+CD25+ Treg cells that are specific for defined artificial autoantigens.27,28 However, CD62L+ and CD62L- Treg cell subpopulations have not been analyzed in these mice yet. We did not detect a difference in the ability of CD4+CD25+CD62L+ and CD62L- subsets to suppress alloresponses in vitro, suggesting that there is no significant difference in alloreactivity between the 2 populations. Based on expression of the memory markers CD44 and CD45RB, CD4+CD25+ Treg cells have been classified as having a memory phenotype.10 We demonstrate in this paper that CD4+CD25+CD62L+ and CD62L- Treg cells differed with regard to expression of CD44 and CD45RB in that CD62L+ Treg cells had intermediate expression levels of these receptors, whereas the CD62L- cells were truly CD44high and CD45RBlow. The previously noted bimodal distribution for CD45RB on CD4+CD25+ Treg cells29 is thus a composite of CD45RB expression on the CD62L+ and CD62L- Treg cell subsets. The differential expression of various memory/activation markers may indicate that the CD62L+ and CD62L- subsets represent distinct stages of CD4+CD25+ Treg cell development30 with functional differences of relevance in vivo beyond trafficking. For example, Fu et al13 reported that CD4+CD25+CD62L+ T cells expanded better than CD4+CD25+CD62L- T cells when stimulated in vitro with anti-CD3 plus IL-2. CD69 is a marker of recent T-cell activation. About 50% of the CD4+CD25+CD62L- T cells were CD69+ compared with only 15% of the CD62L+ subset. It has been demonstrated for total CD4+CD25+ T cells that CD69 expression does not distinguish between cells with and without suppressor function.11 Furthermore, we did not detect significant differences between CD4+CD25+CD62L+ and CD62L- T cells in any of the Treg cell assays performed in vitro. Available data thus do not support the notion that CD4+CD25+CD62L- T cells are contaminated by a major fraction of recently activated conventional T cells.
In conclusion, we have demonstrated that only the CD62L+ subpopulation of donor-type CD4+CD25+ Treg cells protected from lethal aGVHD induced by CD4+CD25- T cells. Both CD4+CD25+CD62L+ and CD4+CD25+CD62L- T cells showed Treg cell characteristics in vitro. Our data suggest that the differential ability of the CD62L+ and CD62L- Treg cell subsets to protect from lethal aGVHD in vivo is due to their differential ability to enter the priming sites of the pathogenic CD4+CD25- donor T cells.
Prepublished online as Blood First Edition Paper, November 16, 2004; DOI 10.1182/blood-2004-05-2044.
Supported by National Institutes of Health grants DK-61295, CA-65237, HL-57443, HL-58250, CA-49605, CA-92225, and AI-37683.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Holger Karsunky and Libu Jerabek for providing congenic mice.


![Figure 4. CD4+CD25+CD62L+ T cells home significantly better to secondary lymphoid tissues than CD4+CD25+CD62L- T cells. Sorted CD4+CD25+CD62L+ (▨) and CD4+CD25+CD62L- T cells (▪) were labeled with 111In before cotransfer with unlabeled CD4+CD25- T cells and TCD BM into irradiated hosts. Mice were killed 48 hours later and radioactivity in the various organs was measured. (A) Global comparison of aGVHD target organs (liver, small bowel, large bowel) and primary lymphoid organs (spleen, peripheral LN [PLN], mesenteric LN [MLN]). Results are expressed as fraction of injected radioactive dose divided by organ weight in grams (% ID/g) representing organ-specific enrichment. Mean + SD is given. (B) Results from individual mice are shown for mesenteric LN, spleen, and liver. The horizontal line represents the group median. P values are provided above the figure. Data were pooled from 3 experiments (CD4+CD25+CD62L+ mice, n = 7; CD4+CD25+CD62L- mice, n = 8).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-05-2044/6/m_zh80050574960004.jpeg?Expires=1768236443&Signature=CPWB-gpBwDeiu0I-hTHe7jpiSVMYzLjb0qFYQcXfat~HopsFOjipM5NTrkHfXzTKfBdu9gMIjiGlg63vfnTjz6aGIvT46eYi3Hlm7Igp6TyXzNgCNG6fHBye1n7RBXxrLNP9xeU8x9Z-LO4CwQK7GkEGpgPIIVY7SJdjgfikjA-jZonaC0zO~k~B8U392MnXubzSrJOtAIYqLg~jUTRGA3Gncr0NEnjUumoH4nnSAb-i6KyKXwynYp3vDUT6vQ4D7jQTMbtNJn8t2C2cEEAg1Zf7KcApK5U5GjBWQsmV5I1vTnfbxD7XIf2-QjLMmutQvOBwwSNthde~hp~JwJwpoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. CD4+CD25+CD62L- T cells are quantitatively less efficient suppressors in vivo. Lethally irradiated BALB/c mice received TCD BM and CD4+CD25- T cells from congenic C57BL/5 without (control hosts) or together with (experimental hosts) CD4+CD25+CD62L+ or CD4+CD25+CD62L- Treg cells from wild-type (WT) C57BL/6 donors. All mice were killed 5 days after transfer. Single cell suspensions were prepared from mesenteric LN (MLN), spleen, and liver of individual mice. Viable cells were counted, stained with appropriate antibodies, and analyzed by flow cytometry. The progeny of CD4+CD25- and CD4+CD25+ T cells was identified as CD4+H-2Kb+[congenic marker]+/-. Results for individual mice are shown. The horizontal line represents the group median. P values are given above the figures. Data were pooled from 4 experiments with 9 to 11 mice per group. (A) Recovery of CD4+CD25+ T cells after transfer of the CD62L+ or CD62L- subset. (B) Expansion of CD4+CD25- T cells in mesenteric LN, spleen, or liver after cotransfer of CD4+CD25+CD62L+ or CD4+CD25+CD62L- Treg cells. (C) Fraction of CD4+CD25+ T-cell progeny among all donor CD4+ T cells in individual mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-05-2044/6/m_zh80050574960005.jpeg?Expires=1768236443&Signature=2H-NWcPXWBkHrdX3gFJTh9t9pMxq0Y8F7kLI3a3qi02f3jQNyAazoE7Ig-rqlms-uPtUPRz8zjP-d4P1YLCufRlHvXuDPYs0XjSDfYg1LwbhtlcF0DYmjP7VzXI~35mkQRoHd3BxE3gCZasHPPCAS7eREUwwFkbVmFeLiOtwnmGKQm2~kfIayWLurGyDL5ufcC-jaCLobONRe1Bf76LZ8~3lpy8tBtzeR8PIFtKeWnTvsMz2uYTRZ~FDAQEmBwSqiMqBZfIS3By2Sy4wIIjb4KDeYeJtNp2iMeb6aK9nA1ODUKgF1QW2BF8sGykPw9FmvXxDNMK3M9xS0PwMiRWHOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal