Comment on Stanzani et al, page 2258
The work by Stanzani and colleagues illustrates the prescience of the statement by Jonathan Swift, “Hobbes clearly proves that every creature lives in a state of war by nature,”1 when applied to weaponry used by opportunistic fungi to survive in mammalian hosts. Aspergillus fumigatus produces a variety of proteases, reactive oxidant scavengers, phospholipases, and hemolysins that mediate penetrating the lung epithelial barrier, nutrient acquisition, and evasion of host defense.
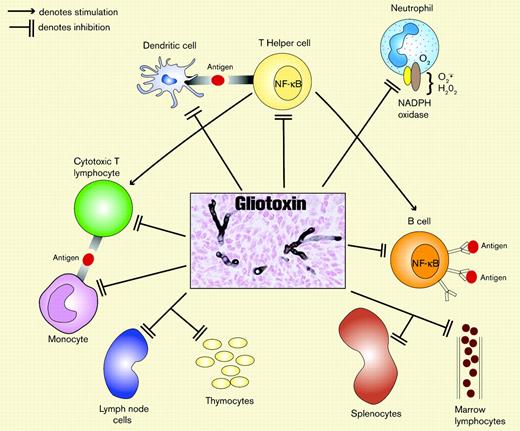
Gliotoxin, an abundant toxin produced by Aspergillus fumigatus (AF) and other fungi, belongs to the epipolythiodioxopiperazine class of metabolites. Gliotoxin has broad immunosuppressive properties that facilitate evasion of host defense (Figure 1). It inhibits phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase assembly and activation, a key component in host defense against filamentous fungi.2 Gliotoxin also inhibits ingestion of AF by macrophages,3 inhibits cytotoxic T-lymphocyte cytotoxicity,3 induces apoptosis of thymocytes, splenocytes, and mesenteric lymph node cells,4 selectively depletes bone marrow of mature lymphocytes in mice, and inhibits activation of nuclear factor kappa B (NF-κB; a transcriptional factor that is a key mediator of cytokine and inflammatory responses) in T and B cells.5
Gliotoxin, a toxin produced by AF, suppresses several key pathways in innate and antigen-specific immunity. Gliotoxin likely suppresses T-cell responses principally by inducing apoptosis of antigen-presenting cells. The center box depicts experimental invasive pulmonary aspergillosis in a mouse (silver stain; original magnification × 400).
Gliotoxin, a toxin produced by AF, suppresses several key pathways in innate and antigen-specific immunity. Gliotoxin likely suppresses T-cell responses principally by inducing apoptosis of antigen-presenting cells. The center box depicts experimental invasive pulmonary aspergillosis in a mouse (silver stain; original magnification × 400).
In this issue of Blood, Stanzani and colleagues made a significant contribution to further understanding the immunosuppressive properties of gliotoxin. They showed that CD4+ and CD8+ T cells from healthy donors had weak cytokine responses to AF crude filtrates, a paradoxical finding given that we are regularly exposed to this ubiquitous organism. Gliotoxin suppressed functional T-cell responses to cytomegalovirus (CMV) and the superantigen staphylococcal enterotoxin B, principally by inducing apoptosis of monocytes and dendritic cells. The effects of gliotoxin in vitro occurred at concentrations below those observed in the serum of patients with invasive aspergillosis. This study highlights the potential immunomodulatory properties of gliotoxin during invasive aspergillosis and raises the possibility of gliotoxin-mediated immunosuppression predisposing such patients to additional opportunistic infections, such as CMV.
Casadevall et al6 employed the term “dual use” virulence factors to describe factors that confer a survival advantage to pathogens both in the environment and in animals. For example, the virulence of Cryptococcus neoformans in animals may have originated from factors that enabled persistence in amoeboid and nematode predators. AF thrives in the environment, but its ecologic niche in humans is highly restrictive, generally only afflicting the severely immunocompromised. It would be useful to understand what other function (if any) gliotoxin serves in the natural environment and whether gliotoxin production correlates with virulence. Potentially, clinical isolates of AF may be more robust gliotoxin producers than environmental strains. Generation of knockout strains deficient in gliotoxin production would be more definitive in evaluating gliotoxin as a virulence factor in experimental aspergillosis and its role in modulating mammalian host defense.
Knowledge gained about gliotoxin may be exploited in the clinic. Circulating gliotoxin levels may be of value in diagnosing Aspergillus infection. Gliotoxin is also a promising target for drug development, and generation of neutralizing antibodies against gliotoxin may be of value in attenuating fungal virulence. In the Hobbesian vision, the more tools we have in our armamentarium, the better. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal