Abstract
Idiopathic pneumonia syndrome (IPS) is a major cause of mortality following allogeneic stem cell transplantation (allo-SCT). Clinical and experimental data support a role for conditioning-induced inflammation and alloreactive T-cell responses in IPS pathophysiology, but the mechanisms by which donor leukocytes are ultimately recruited to the lung are not fully understood. RANTES is a chemokine ligand that is up-regulated during inflammation and promotes the recruitment of T cells and macrophages to sites of tissue damage. Using a lethally irradiated murine SCT model (B6 → B6D2F1), we evaluated the role of donor leukocyte–derived RANTES in the development of IPS. Pulmonary mRNA and protein levels of RANTES were significantly elevated in allo-SCT recipients compared to syngeneic controls and were associated with enhanced mRNA expression of CCR5 and CCR1 and with inflammatory cell infiltration into the lung. Allo-SCT with RANTES-/- donor cells significantly decreased IPS and improved survival. Combinations of allogeneic wild-type or RANTES-/- bone marrow with wild-type or RANTES-/- T cells demonstrated that the expression of RANTES by donor T cells was critical to the development of lung injury after SCT. These data reveal that donor T cells can help regulate leukocyte recruitment to the lung after allo-SCT and provide a possible mechanism through which inflammation engendered by SCT conditioning regimens is linked to allo-specific T-cell responses during the development of IPS.
Introduction
Allogeneic stem cell transplantation (allo-SCT) is an important therapy for a number of malignant and nonmalignant diseases. The broader application of allo-SCT is limited by several complications, including the development of graft-versus-host disease (GVHD) and pulmonary toxicity. Diffuse lung injury can occur in 25% to 55% of allo-SCT recipients.1-6 In approximately 50% of patients, infectious organisms are not identified, and these cases have been defined as idiopathic pneumonia syndrome (IPS). IPS is associated with mortality rates of more than 70% despite the use of high-dose steroids, broad-spectrum antimicrobial agents, and aggressive supportive measures.1,4 This form of lung injury is characterized by complex pathophysiology involving cytotoxicity from irradiation and chemotherapy,7,8 the production of inflammatory cytokines,9-14 and the recruitment of both donor T cells and accessory cells.12,15-17
The expression of several chemokines is also increased in the lung after allogeneic SCT,18-21 and recent data from our group have shown mechanistic links between specific chemokine receptor-to-ligand interactions and the recruitment of donor T cells, monocytes, and macrophages to the lung during IPS.19,21 Regulated on activation, normal T-cell–expressed and secreted (RANTES/CCL5) is a member of the CC chemokine family of proteins that is strongly chemoattractant for activated T cells, monocytes, eosinophils, and basophils.22,23 Increased RANTES expression has been shown in a number of experimental systems, including those modeling transplantation rejection,24 sclerodermatous GVHD,25 and acute lung injury after allo-SCT.18-20 RANTES can be produced by several cell types including fibroblasts,26 epithelial27-29 and endothelial cells,30,31 activated T cells,32 and macrophages,33 and its expression can be induced by proinflammatory cytokines including tumor necrosis factor α (TNFα), interleukin-1 β (IL-1β), and interferon γ (IFNγ).20,26-29,31 CCR1 and CCR5 are the primary receptors for RANTES. These receptors are expressed on a variety of cells including activated Th1/Tc1 lymphocytes, macrophages, and immature dendritic cells, but neither receptor binds exclusively to RANTES.34
We used a well-established murine SCT model, wherein IPS develops following myeloablative conditioning and in the context of major and minor histocompatibility differences between donor and recipient, to study the role of RANTES of leukocyte infiltration into the lung. IPS in this system is dependent upon the infusion of allogeneic T cells, along with the pretransplant radiation dose,35 and involves the recruitment of alloreactive CD4+ and CD8+ T cells to the lung.19,20,35 Although the severity of IPS is exacerbated by the addition of cytoxan to total body irradiation (TBI) in a related SCT model,16 the effects of “chemotherapy only” conditioning regimens on the development of IPS have yet to be explored in this system. Importantly, lung injury that develops in this model is defined by several features that characterize human disease, including robust pulmonary infiltrates, increased capillary permeability, altered pulmonary physiology, and inflammatory cytokine release.19,20 Using this system, we demonstrate that the development of IPS is associated with increases in lung mRNA and bronchoalveolar lavage fluid (BALF) protein levels of RANTES that coincide with elevated pulmonary expression of CCR1 and CCR5. Furthermore, our data specifically show that RANTES secretion by donor T cells contributes to IPS and that the use of RANTES-deficient T cells results in a significant reduction in lung injury after allogeneic SCT, regardless of the phenotype of the coadministered bone marrow cells.
Materials and methods
Mice and stem cell transplantation
Female C57BL/6 (H-2b, CD45.2+), B6 Ly5.2 (H-2b, CD45.1+), B6D2F1 (H2bxd) mice were purchased from The Jackson Laboratory (JAX; Bar Harbor, ME) or from the Frederick Cancer Research and Development Center (National Cancer Institute; Frederick, MD). RANTES-/- mice (H-2b B6.129P2-Cc15tmlHSO), kindly provided by Dr Jonathan Serody, were backcrossed onto a B6 background for 6 generations.36 Recipient animals used for SCT and animals used for in vitro experiments were between 10 and 14 weeks old. Donor animals were between 10 and 30 weeks old, and appropriate age-matched controls were used. The University of Michigan Committee on the Use and Care of Animals (UCUCA) approved all experiments.
Mice received transplants according to a standard protocol as previously described.37 Briefly, B6D2F1 mice received cell mixtures of 5 × 106 bone marrow (BM) cells supplemented with 1.8 to 2.0 × 106 splenic T cells from either syngeneic (B6D2F1) or allogeneic C57BL/6, B6 Ly5.2, or B6 RANTES-/- donors. T-cell purification was performed by magnetic bead separation using CD4 and CD8 MicroBeads and the autoMACS system (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's protocol, with more than 85% of cells obtained being positive for CD4 or CD8 surface antigens (data not shown). Percentages of purified CD4+ and CD8+ T cells did not significantly differ between donors. Prior to transplantation, host mice received 11 or 13 Gy of TBI (137Cs source) delivered in 2 fractions separated by 3 hours to reduce gastrointestinal toxicity. Mice were subsequently housed in sterilized micro-isolator cages and received normal chow and autoclaved hyperchlorinated water for the first 3 weeks after SCT and filtered water thereafter.
Bronchoalveolar lavage (BAL) and cell surface phenotyping
At the time of analysis, mice were killed by exsanguinations, and BAL was performed as previously described.38 Supernatant from the first lavage was frozen for subsequent analysis of cytokine/chemokine content. Cell pellets were combined, washed, and counted. To analyze cell surface phenotype, aliquots of cell suspensions were stained with fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies (MoAbs) to CD4, CD8, CD45.1, CD45.2, GR-1, CD11b; phycoerythrin (PE)–conjugated MoAbs to CD4, CD8, F4/80; or allophycocyanin (APC)–conjugated MoAbs to CD4 and CD8 for flow cytometric analysis as previously described.10 All MoAbs were purchased from BD Biosciences Pharmingen (San Diego, CA). Monocyte/macrophage counts were determined by subtracting the sum counts of CD4+, CD8+, and GR-1+ cells from the total BALF counts. B220+ B lymphocytes represent only 1% of total cells after syngeneic or allogeneic SCT and were not separately differentiated (data not shown).
Intracellular cytokine analysis
For intracellular RANTES staining, BAL cells were stimulated with the leukocyte activation cocktail in the presence of GolgiPlug (BD Biosciences Pharmingen) for 4 hours and then stained using reagents from the intracellular cytokine staining kit (BD Biosciences Pharmingen) according to the manufacturer's protocol. RANTES staining was performed using either unlabeled rabbit anti-RANTES (mouse) Abs (Serotec, Raleigh, NC) or rabbit isotype IgG control Abs as primary antibodies and subsequent detection using FITC-conjugated goat anti–rabbit immunoglobulin G (IgG) secondary antibody (Zymed Laboratories, San Francisco, CA). Three-color flow cytometric analysis of 1 × 104 cells was performed using a FACSCalibur (BD Biosciences Pharmingen). The FACScan was calibrated using FITC-, PE-, and APC-conjugated, nonspecific IgG antibodies.
Semiquantitative histopathology of lung, liver, and intestine
Pulmonary toxicity was assessed in SCT recipients by examination of lung histopathology as previously described.38 Hematoxylin-eosin–stained lung sections from individual mice were coded without reference to mouse type or prior treatment regimen and independently examined by C.L. to establish an index of injury. Lung tissue was evaluated for the presence of periluminal infiltrates (around airways and vessels) or parenchymal pneumonitis (involving the alveoli or interstitium) using a previously described semiquantitative scoring system that incorporates both the severity (periluminal infiltrates: 0 = no infiltrates, 1 = 1 to 3 cell diameters thick, 2 = 4 to 10 cell diameters thick, 3 = > 10 cell diameters thick; pneumonitis: 0 = no infiltrates, 1 = increased cells only visible at high magnification [× 400], 2 = easily seen cellular infiltrate or interstitial thickening, 3 = consolidation by inflammatory cells and interstitial thickening) and extent (percentage of lung tissue involved: 5% to 25% = 1; > 25% to 50% = 2; > 50% = 3) of histopathology.38 Photographs of histopathology were taken with an Olympus BX40 microscope (20 ×/0.4 lens) (Olympus, Tokyo, Japan) as digital images (camera: JVC-QX5HDU; Victor Company of Japan, Yokohama, Japan) using Leica IM50 imaging manager and acquisition software (Leica Microsystems, Bannockburn, IL) and further processed with Adobe Photoshop (Adobe, San Jose, CA).
Detailed gastrointestinal (GI) tract and liver histopathologic analysis was performed in a blinded fashion by C.L. 42 days after transplantation, as previously described.39-41 Specifically, 7 parameters each were scored for small bowel (villous blunting, crypt regeneration, crypt epithelial cell apoptosis, crypt loss, luminal sloughing of cellular debris, lamina propria inflammatory cell infiltrate, and mucosal ulceration), large bowel (crypt regeneration, crypt epithelial cell apoptosis, crypt loss, surface colonocyte vacuolization, surface colonocyte attenuation, lamina propria inflammatory cell infiltrate, and mucosal ulceration), and 10 parameters for liver (portal tract expansion by an inflammatory cell infiltrate, lymphocytic infiltrate of bile ducts, bile duct epithelial cell apoptosis, bile duct epithelial cell sloughing, vascular endothelialitis, parenchymal apoptosis, parenchymal microabscesses, parenchymal mitotic figures, hepatocellular cholestasis, and hepatocellular steatosis). The scoring system for each parameter denoted 0 as normal, 0.5 as focal and rare, 1 as focal and mild, 2 as diffuse and mild, 3 as diffuse and moderate, and 4 as diffuse and severe.
Clinical GVHD and survival
Survival was monitored daily, and GVHD clinical scores were assessed weekly by a scoring system incorporating 5 clinical parameters: weight loss, posture (hunching), mobility, fur texture, and skin integrity, as previously described.38
RNase protection assay
Determination of mRNA expression by the RNase protection assay (RPA) was completed as previously described using the multiprobe-template sets mCK-5b and mCR-5 purchased from BD Biosciences Pharmingen.19 [32P]UTP-radiolabeled antisense riboprobes were synthesized according to the manufacturer's protocol and purified using G-25 Sephadex Quick Spin Columns (Roche, Indianapolis, IN). Expression of chemokine genes was quantified by RPA using RiboQuant RPA kits (BD Biosciences Pharmingen), according to the manufacturer's protocol. Protected RNA products were separated on a 5% polyacrylamide gel; the gel was exposed to a storage phosphor screen (Molecular Dynamics, Sunnyvale, CA) for quantification. Signal intensity was measured with ImageQuant (Molecular Dynamics) and standardized to the intensity of the L32 signal for each sample.
Measurement of cytokine and chemokine protein levels by ELISA
Concentrations of RANTES were measured in BAL fluid and serum using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN). Assays were performed according to the manufacturer's protocol. ELISA plates were read by microplate reader (Bio-Rad Laboratories, Hercules, CA).
Immunoneutralization of RANTES
Polyclonal Abs against RANTES were generated in multiple-site immunized New Zealand White rabbits using the peptide sequence NH2-GCAYLSLELPRAHVKEYFYT-OH for RANTES (Bio-Synthesis, Lewisville, TX). The specificity of the anti-RANTES antibodies was rigorously screened before its use in SCT experiments, and it lacked cross-reactivity with other chemokines including Mip-1α, MCP-1, KC, Mip-2, Mig, and IP-10 as determined by ELISA. The neutralizing capacity was confirmed by performing in vitro migration studies (as described under “In vitro cell migration assay”). Allogeneic SCT recipients were injected intraperitoneally with 250 μL of anti-RANTES polyclonal Abs or with 250 μL control rabbit serum on days 0, 2, 5, and then every third day SCT until the time of analysis. Syngeneic SCT recipients received control serum only.
In vitro cell migration assay
Recombinant murine RANTES (mRANTES) was purchased from Pepro-Tech (Rocky Hill, NJ). mRANTES was preincubated with anti-RANTES polyclonal antibodies or control for 3 hours at room temperature at a concentration of 25 ng/mL, and 600 μL of each combination was subsequently placed in the lower chamber of a 24-well transwell migration plate (Corning Costar, Corning, NY). Some wells were filled with control serum alone to determine the basal migration rate. Next, 5 × 105 naive splenic T lymphocytes were resuspended in 100 μL of either anti-RANTES polyclonal Abs or control (without chemokine) and placed in the upper chamber of transwell inserts (6.5 mm diameter; 5-μm pore size) (Corning Costar). Inserts were placed in the corresponding lower wells containing control serum alone (basal), control serum plus chemokine (positive control), or polyclonal Abs to RANTES plus chemokine (experimental group). After 4 hours of migration at 37°C, inserts were removed, and the sera in the lower chambers were collected. The wells were then thoroughly washed with 1 mL of media (× 5), and aliquots were combined with the first collection sample. The numbers of cells that migrated to the lower chamber were determined by using a Z1 Coulter Counter (Beckman Coulter, Fullerton, CA). The percentage of cells that migrated from the upper into the lower chamber was calculated and ultimately averaged from triplicate wells.
Statistical considerations
All values are expressed as the mean ± standard error of the mean (SEM). Statistical comparisons between groups were completed using the parametric independent sample t test if n > 5 animals per group and using the Mann-Whitney test if n < 5 animals per group. The Wilcoxon rank test was used for analyzing survival data.
Results
Enhanced pulmonary expression of RANTES correlates with the initial influx of donor T cells into the lungs after allogeneic SCT
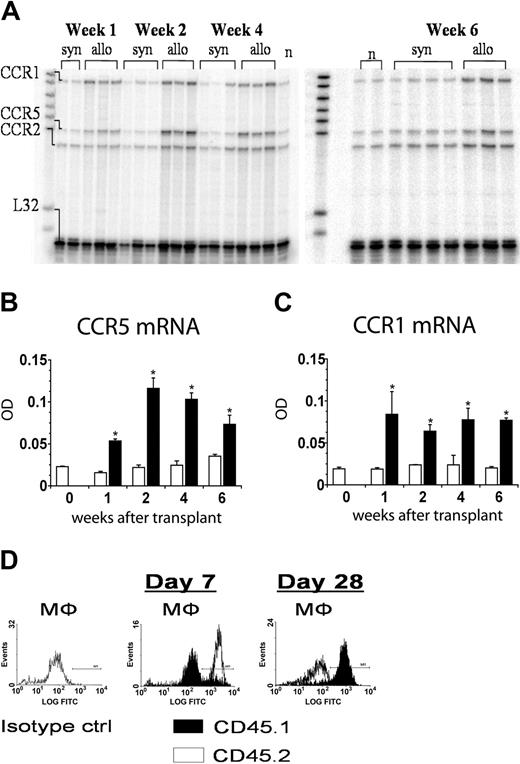
Lethally (1300 cGy split dose) irradiated B6D2F1 mice received SCT from either syngeneic (B6D2F1) or allogeneic (B6 CD45.1+) donors as described in “Materials and methods.” RNA was isolated from the lungs of animals who received transplants on days 2, 7, 14, 28, and 42 after transplantation, and mRNA levels for RANTES were determined by RNase protection assay. RANTES mRNA expression was low 2 days after allo-SCT and indistinguishable from syngeneic controls (Figure 1A), whereas RANTES protein expression was not detectable in either group (data not shown). BALF cellularity was also low in both groups at this time, and donor-derived T cells were not yet detectable in allo-SCT recipients (Figure 1A). By contrast, mRNA levels of RANTES were significantly increased in allo-SCT recipients by day 7, remained elevated compared to syngeneic controls at all subsequent time points, and correlated with elevated levels of RANTES protein in the BALF on days 14 (277.7 ± 21.2 vs 18.2 ± 18.2 pg/mL) and 42 (103.3 ± 40.1 vs 0.8 ± 0.8 pg/mL) (Figure 1B). Of particular note, the initial increase in RANTES mRNA expression seen after allo-SCT correlated with the influx of donor-derived T cells into the lungs of these animals as early as day 7 (98% CD8+ and 94% CD4+ percentage donor versus 2% CD8+ and 6% CD4+ host) through day 14 (Figure 1C).
Enhanced pulmonary RANTES expression correlates with the influx of donor T cells into the lung after allogeneic SCT. Lethally irradiated B6D2F1 mice received SCT from either syngeneic B6D2F1 (□) or allogeneic B6 (▪) donors. RNA was isolated from the lungs of animals on days 2, 7, 14, 28, and 42 after transplantation, and RANTES expression was determined by RNase protection assay. Protein levels of RANTES present in BALF were determined by ELISA. On days 2, 7, and 14, T-cell chimerism was determined in the BALF of mice that received transplants using flow cytometry and antibodies to CD45.1 (donor marker) and CD45.2 (host marker). (A) Two days after SCT, RANTES mRNA expression in the lung is low in each SCT group and correlates with low BALF cellularity and with the lack of donor T cells in the bronchoalveolar space. (B) RANTES mRNA expression is increased by day 7 after allo-SCT, remains elevated compared to syngeneic controls throughout the observation period, and is associated with increases in BALF protein expression on days 14 and 42. (C) The early rise in RANTES mRNA expression correlates with the influx of donor-derived T cells by day 7 (see first paragraph under “Results”) and day 14 after SCT. Data are presented as mean ± SEM; n = 3 to 10 animals per group; *P < .05, (▪, allogeneic) versus (□, syngeneic).
Enhanced pulmonary RANTES expression correlates with the influx of donor T cells into the lung after allogeneic SCT. Lethally irradiated B6D2F1 mice received SCT from either syngeneic B6D2F1 (□) or allogeneic B6 (▪) donors. RNA was isolated from the lungs of animals on days 2, 7, 14, 28, and 42 after transplantation, and RANTES expression was determined by RNase protection assay. Protein levels of RANTES present in BALF were determined by ELISA. On days 2, 7, and 14, T-cell chimerism was determined in the BALF of mice that received transplants using flow cytometry and antibodies to CD45.1 (donor marker) and CD45.2 (host marker). (A) Two days after SCT, RANTES mRNA expression in the lung is low in each SCT group and correlates with low BALF cellularity and with the lack of donor T cells in the bronchoalveolar space. (B) RANTES mRNA expression is increased by day 7 after allo-SCT, remains elevated compared to syngeneic controls throughout the observation period, and is associated with increases in BALF protein expression on days 14 and 42. (C) The early rise in RANTES mRNA expression correlates with the influx of donor-derived T cells by day 7 (see first paragraph under “Results”) and day 14 after SCT. Data are presented as mean ± SEM; n = 3 to 10 animals per group; *P < .05, (▪, allogeneic) versus (□, syngeneic).
The pulmonary expression of RANTES is associated with the up-regulation of CCR5 and CCR1 and with the recruitment of inflammatory cells into the lung after allogeneic SCT
CCR5 and CCR1 are the primary receptors for RANTES and can be expressed on a variety of effector cells, including macrophages, that have been shown to contribute to experimental IPS.10,19 RNA was isolated from the lungs of animals on days 7, 14, 28, and 42 after transplantation, and CCR5 and CCR1 expression was determined by RNase protection assay. Increases in pulmonary RANTES mRNA levels after allo-SCT were associated with the elevated expression of CCR5 and CCR1 mRNA in the lungs of these animals compared to syngeneic controls (Figure 2A-C). Changes in chemokine receptor expression coincided with the influx of donor-derived macrophages into the lung; flow cytometry using antibodies to CD45.1 (donor marker) and CD45.2 (host marker) and forward-side scatter characteristics for pulmonary macrophages revealed that only 10% of macrophages were of donor origin on day 7, and turnover was not complete until 4 weeks after SCT (Figure 2D). Similar finding were noted when BALF cells were costained with antibodies to the cell surface marker F4/80 and either CD45.1 or CD45.2 (data not shown). Moreover, the enhanced expression of both CCR5 and CCR1 ultimately reflected increases in inflammatory cell infiltration into both the bronchoalveolar space and the lung parenchyma of allo-SCT recipients, consistent with the evolution of lung injury observed in this experimental model (Table 1).19,20
CCR5 and CCR1 mRNA expression is increased after allogeneic SCT and is associated with the influx of donor T cells and macrophages into the bronchoalveolar space. RNA was isolated from the lungs of animals on days 7, 14, 28, and 42 after transplantation, and CCR5 and CCR1 expression was determined by RNase protection assay as described in Figure 1. (A-C) Pulmonary CCR5 and CCR1 mRNA expression were significantly increased after allo-SCT at all time points when compared to syngeneic controls. On days 7, 14, and 28 after transplantation, BALF monocyte/macrophage chimerism was determined by flow cytometry using antibodies to CD45.1 (donor marker, ▪) and CD45.2 (host marker, □) and either forward-side scatter characteristics for pulmonary macrophages (D) or costaining for the F4/80 cell surface marker (data not shown). Data are presented as mean ± SEM; n = 3 to 10 animals per group; *P < .05, (▪, allogeneic) versus (□, syngeneic).
CCR5 and CCR1 mRNA expression is increased after allogeneic SCT and is associated with the influx of donor T cells and macrophages into the bronchoalveolar space. RNA was isolated from the lungs of animals on days 7, 14, 28, and 42 after transplantation, and CCR5 and CCR1 expression was determined by RNase protection assay as described in Figure 1. (A-C) Pulmonary CCR5 and CCR1 mRNA expression were significantly increased after allo-SCT at all time points when compared to syngeneic controls. On days 7, 14, and 28 after transplantation, BALF monocyte/macrophage chimerism was determined by flow cytometry using antibodies to CD45.1 (donor marker, ▪) and CD45.2 (host marker, □) and either forward-side scatter characteristics for pulmonary macrophages (D) or costaining for the F4/80 cell surface marker (data not shown). Data are presented as mean ± SEM; n = 3 to 10 animals per group; *P < .05, (▪, allogeneic) versus (□, syngeneic).
Progression of IPS after allo-SCT
. | Lung pathology score, mean ± SEM . | . | Total BALF cellularity, × 106, mean ± SEM . | . | BALF T cells, × 106, mean ± SEM . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Week . | Syngeneic . | Allogeneic . | Syngeneic . | Allogeneic . | Syngeneic . | Allogeneic . | |||
| 1 | 0 ± 0 | 0 ± 0 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.003 ± 0.001 | 0.2 ± 0.1* | |||
| 2 | 0.1 ± 0.1 | 0.8 ± 0.1* | 0.3 ± 0.1 | 1.5 ± 0.3* | 0.009 ± 0.001 | 0.7 ± 0.1* | |||
| 4 | 0.1 ± 0.1 | 3.8 ± 0.3* | 0.4 ± 0.1 | 2.5 ± 0.3* | 0.026 ± 0.008 | 1.0 ± 0.2* | |||
| 6 | 0.3 ± 0.1 | 6.7 ± 0.4* | 0.8 ± 0.1 | 2.5 ± 0.4* | 0.140 ± 0.040 | 1.2 ± 0.3* | |||
. | Lung pathology score, mean ± SEM . | . | Total BALF cellularity, × 106, mean ± SEM . | . | BALF T cells, × 106, mean ± SEM . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Week . | Syngeneic . | Allogeneic . | Syngeneic . | Allogeneic . | Syngeneic . | Allogeneic . | |||
| 1 | 0 ± 0 | 0 ± 0 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.003 ± 0.001 | 0.2 ± 0.1* | |||
| 2 | 0.1 ± 0.1 | 0.8 ± 0.1* | 0.3 ± 0.1 | 1.5 ± 0.3* | 0.009 ± 0.001 | 0.7 ± 0.1* | |||
| 4 | 0.1 ± 0.1 | 3.8 ± 0.3* | 0.4 ± 0.1 | 2.5 ± 0.3* | 0.026 ± 0.008 | 1.0 ± 0.2* | |||
| 6 | 0.3 ± 0.1 | 6.7 ± 0.4* | 0.8 ± 0.1 | 2.5 ± 0.4* | 0.140 ± 0.040 | 1.2 ± 0.3* | |||
B6D2F1 mice received syngeneic or allogeneic SCT as in Figure 1. Recipients of allogeneic SCT develop significant lung pathology and alterations in total BALF cellularity and T-cell counts compared to syngeneic controls. Data presented are from 2 experiments representative of 3 and are presented as mean ± SEM; n = 8 to 10 per group per time point.
P < .01
Allogeneic SCT with RANTES-deficient donor mice results in a significant reduction in IPS severity and in improved survival after allo-SCT
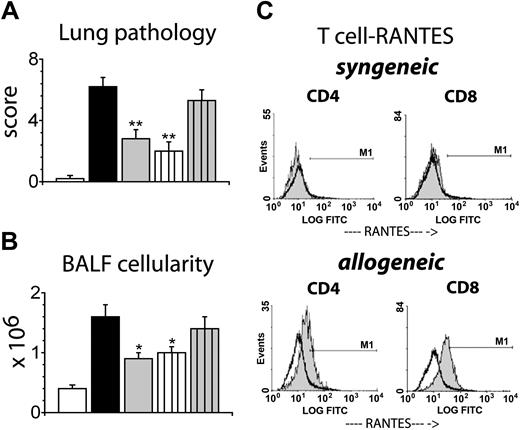
Since RANTES can be secreted both by hematopoietic32,33 and nonhematopoietic26-31 cells, donor-derived (hematopoietic) and host-derived (nonhematopoietic) cells may contribute to the increase in RANTES expression seen in the lung after allogeneic SCT. However, RPA data revealed that the initial rise in RANTES mRNA expression coincided with the arrival of donor T cells to the lung. Therefore, we hypothesized that donor-derived RANTES would contribute to the development of IPS. To test this hypothesis, B6D2F1 mice were again conditioned with 1300 cGy and received SCT from either syngeneic (B6D2F1), allogeneic wild-type B6, or allogeneic RANTES-deficient (RANTES-/-) B6 donors. Animals were analyzed at 4 and 6 weeks after SCT for lung histology along with BALF cellularity and cellular phenotypes. As shown in Figure 3, lungs harvested from syngeneic SCT recipients remained essentially normal, but recipients of allo-SCT from wild-type B6 donors developed significant lung histopathology that was characterized by a dense mononuclear infiltrate around both bronchial and vascular structures and interstitial inflammation (Figure 3A). By contrast, allogeneic SCT from RANTES-/- donors resulted in a significant reduction of lung pathology and BALF cellularity at weeks 4 and 6 after SCT compared to wild-type controls (Figure 3A-C). Specifically, the numbers of CD8+ T cells and monocytes/macrophages were significantly decreased (Figure 3E-F), whereas numbers of CD4+ T cells and GR-1+ granulocytes also were reduced but did not reach statistical significance. This protective effect was associated with improved overall survival (week 6, 83% vs 54%) after allo-SCT, but GVHD-related scores for the liver and the large and small bowel were not significantly different between allogeneic groups (Figure 4).
Allogeneic SCT with RANTES-deficient donor cells results in significantly decreased IPS severity. Lethally irradiated B6D2F1 mice received SCT from either syngeneic B6D2F1 (□), or allogeneic B6 wild-type (▪) or B6 RANTES-/- (▦) donors. Animals that received transplants were analyzed at 4 and 6 weeks after SCT. (A) Photomicrographs of lung tissue 6 weeks after SCT (HE; × 200). (B) Lung injury as assessed by a semiquantitative scoring index at 4 and 6 weeks after SCT. Data are presented as mean ± SEM and are combined from 2 comparable experiments at week 6 (n = 9 to 11 per group; *P < .01) or from 1 experiment at week 4 (n = 4-8 per group; *P < .05). BALF cellularity (C), CD4+ T cells (D), CD8+ T cells (E), monocytes/macrophages (F), and GR-1+ cells (G) 4 and 6 weeks after SCT. Data are expressed as mean ± SEM and are either combined from 2 comparable experiments (n = 9 to 11 per group) at week 6 or from 1 experiment (n = 4 to 8 per group) at week 4; *P < .05.
Allogeneic SCT with RANTES-deficient donor cells results in significantly decreased IPS severity. Lethally irradiated B6D2F1 mice received SCT from either syngeneic B6D2F1 (□), or allogeneic B6 wild-type (▪) or B6 RANTES-/- (▦) donors. Animals that received transplants were analyzed at 4 and 6 weeks after SCT. (A) Photomicrographs of lung tissue 6 weeks after SCT (HE; × 200). (B) Lung injury as assessed by a semiquantitative scoring index at 4 and 6 weeks after SCT. Data are presented as mean ± SEM and are combined from 2 comparable experiments at week 6 (n = 9 to 11 per group; *P < .01) or from 1 experiment at week 4 (n = 4-8 per group; *P < .05). BALF cellularity (C), CD4+ T cells (D), CD8+ T cells (E), monocytes/macrophages (F), and GR-1+ cells (G) 4 and 6 weeks after SCT. Data are expressed as mean ± SEM and are either combined from 2 comparable experiments (n = 9 to 11 per group) at week 6 or from 1 experiment (n = 4 to 8 per group) at week 4; *P < .05.
The reduction in IPS severity following SCT with RANTES-deficient donor cells is associated with improved survival without significant decreases in GVHD target organ pathology. Lethally irradiated B6D2F1 mice received SCT from either syngeneic B6D2F1 (□), or allogeneic B6 wild-type (▪) or B6 RANTES-/- (▦) donors as described in Figure 3. Animals that received transplants were monitored daily for survival (A). At week 6, histopathology of the liver (B) along with the small and large bowel (C) were assessed for GVHD severity using a semiquantitative scoring system specific for each tissue. Data are expressed as mean ± SEM and are combined from 2 comparable experiments, n = 10 to 28 per group for survival data and n = 9 to 14 per group for pathology data; *P < .06.
The reduction in IPS severity following SCT with RANTES-deficient donor cells is associated with improved survival without significant decreases in GVHD target organ pathology. Lethally irradiated B6D2F1 mice received SCT from either syngeneic B6D2F1 (□), or allogeneic B6 wild-type (▪) or B6 RANTES-/- (▦) donors as described in Figure 3. Animals that received transplants were monitored daily for survival (A). At week 6, histopathology of the liver (B) along with the small and large bowel (C) were assessed for GVHD severity using a semiquantitative scoring system specific for each tissue. Data are expressed as mean ± SEM and are combined from 2 comparable experiments, n = 10 to 28 per group for survival data and n = 9 to 14 per group for pathology data; *P < .06.
In vivo neutralization of RANTES reduces the severity of IPS after allo-SCT
The results using RANTES-deficient mice as SCT donors were intriguing and suggested that RANTES-specific immunoneutralization strategies would also be effective in reducing the severity of IPS. We therefore generated polyclonal antibodies to RANTES using the peptide sequence NH2-GCAYLSLELPRAHVKEYFYTOH. In our first set of experiments, we determined whether the antibodies we developed effectively inhibited T-cell migration in vitro. Freshly isolated splenic T cells were allowed to migrate to RANTES through transwell inserts (5-μm pores) for 4 hours in the presence or absence of polyclonal antibodies against RANTES and compared to basal migration without a chemokine stimulus. The proportion of migrated cells was calculated as a fraction of the input population, defined as percent chemotaxis, and then averaged from triplicate wells. As depicted in Figure 5A, significant migration was observed in response to RANTES stimulation when T cells were suspended in control serum. In contrast, RANTES-induced migration was significantly reduced in the presence of the RANTES polyclonal antibodies and did not differ from the basal rate of migration seen in the absence of RANTES (Figure 5A).
Immunoneutralization of RANTES reduces T-cell migration in vitro and the severity of IPS after allogeneic SCT. (A) Freshly isolated splenic T cells were allowed to migrate to RANTES through transwell inserts (5-μm pores) for 4 hours in the presence (▦) or absence (▪) of polyclonal antibodies against RANTES and compared to basal migration without a chemokine stimulus (□). Data presented are from 1 of 3 comparable experiments; *P < .05. Lethally irradiated B6D2F1 mice received allogeneic wild-type SCT and either control serum (▪) or anti-RANTES polyclonal antibodies (▦). Syngeneic animals received control serum only (□). Lung injury was assessed by pathology score (B) and BALF cellularity (C) 6 weeks following allo-SCT. Data are presented as mean ± SEM and are pooled from 2 similar experiments; n = 6 to 8 per group; *P < .05.
Immunoneutralization of RANTES reduces T-cell migration in vitro and the severity of IPS after allogeneic SCT. (A) Freshly isolated splenic T cells were allowed to migrate to RANTES through transwell inserts (5-μm pores) for 4 hours in the presence (▦) or absence (▪) of polyclonal antibodies against RANTES and compared to basal migration without a chemokine stimulus (□). Data presented are from 1 of 3 comparable experiments; *P < .05. Lethally irradiated B6D2F1 mice received allogeneic wild-type SCT and either control serum (▪) or anti-RANTES polyclonal antibodies (▦). Syngeneic animals received control serum only (□). Lung injury was assessed by pathology score (B) and BALF cellularity (C) 6 weeks following allo-SCT. Data are presented as mean ± SEM and are pooled from 2 similar experiments; n = 6 to 8 per group; *P < .05.
In light of these encouraging in vitro results, we next determined the effects of in vivo neutralization of RANTES on the development of IPS. B6D2F1 recipient mice received either syngeneic or allogeneic wild-type SCT. Allo-SCT recipients were injected intraperitoneally with 250 μL of either polyclonal anti-RANTES antibodies or control serum from the day of transplantation until the time point of analysis 6 weeks after transplantation. We chose this schedule to block RANTES during the entire time period of elevated pulmonary RANTES mRNA levels. Syngeneic controls received preimmune serum at the same volume and schedule. All groups were analyzed by week 6 for lung histopathology and BALF cellularity. Consistent with previous experiments in this system, allo-SCT recipients receiving control serum developed significant lung injury, whereas immunoneutralization of RANTES resulted in a significant reduction in both lung pathology and BALF cellularity (Figure 5B). The administration of rabbit serum had no effect on lung histopathology in syngeneic controls, nor were symptoms of serum sickness observed (data not shown).
RANTES production by donor T cells, but not of bone marrow–derived accessory cells, is critical to the development of lung injury after allogeneic SCT
Data in Figure 3 demonstrate a role for donor-derived RANTES in the development of IPS. To more closely evaluate the critical cellular source of RANTES, lethally irradiated (1100 cGy) B6D2F1 received either wild-type B6 bone marrow supplemented with RANTES-/- B6 T cells or RANTES-/- B6 bone marrow supplemented with wild-type B6 T cells. Syngeneic and allogeneic wild-type controls along with recipients of complete RANTES-/- SCT were again included, and the severity of IPS was analyzed at week 6. As shown in Figure 5, transplantation of RANTES-/- T cells had a significant impact on IPS severity regardless of the phenotype of the coadministered BM cells. Not only was the mean lung histopathology score significantly decreased in recipients of B6 wild-type BM and B6 RANTES-/- T cells compared to wild-type allogeneic controls (2.0 ± 0.6 vs 6.2 ± 0.6), but it also was reduced to the level measured in recipients of RANTES-deficient bone marrow and T cells (2.8 ± 0.6) (Figure 6A-B). In contrast, the development of lung injury was not affected in animals receiving wild-type T cells and RANTES-/- bone marrow cells (5.3 ± 0.7). These findings were complimented by a final set of experiments, wherein the intracellular expression of RANTES in pulmonary T cells was measured by flow cytometric analysis 14 days following SCT. As shown in Figure 6C, intracellular RANTES expression was significantly higher in CD4+ and CD8+ T cells isolated from the lungs of allo-SCT recipients when compared to syngeneic controls (mean intensity, 7.5 ± 0.5 [syn] vs 15.4 ± 2.0 [allo] for CD4+ T cells, and 7.3 ± 0.4 [syn] vs 22.5 ± 3.3 for CD8+ T cells). Collectively, these data demonstrate that donor T-cell–derived RANTES plays an important role in the development of lung injury after allo-SCT.
RANTES deficiency of donor T cells, but not of bone marrow–derived accessory cells, mediates reduced IPS severity after allogeneic SCT. Lethally irradiated B6D2F1 mice received SCT from either syngeneic B6D2F1 (□) or allogeneic B6 wild-type (▪) or RANTES-/- (▦) donors as described in Figure 3. In addition, groups of B6D2F1 animals received wild-type B6 bone marrow and RANTES-/- B6 T cells (white lined bar) or RANTES-/- B6 bone marrow and wild-type B6 T cells (gray lined bar). The severity of IPS was subsequently assessed in animals that received transplants by measuring lung histopathology (A) and BALF cellularity (B) 6 weeks after SCT. Data are presented as mean ± SEM and are from 1 experiment (pathology; n = 4 to 9 per group, **P < .01) or from 2 experiments (BAL cellularity; n = 8 to 17 per group, *P < .05. (C) Intracellular RANTES expression in T cells isolated from the lungs 2 weeks after SCT. Mean intensity equals 7.5 ± 0.5 (syn) vs 15.4 ± 2.0 (allo) for CD4+ cells and mean intensity equals 7.3 ± 0.4 (syn) vs 22.5 ± 3.3 (allo) for CD8+ cells. Data presented are from 1 of 2 comparable experiments; solid gray indicates RANTES, thin black line unfilled indicates isotype control; n = 3 per group.
RANTES deficiency of donor T cells, but not of bone marrow–derived accessory cells, mediates reduced IPS severity after allogeneic SCT. Lethally irradiated B6D2F1 mice received SCT from either syngeneic B6D2F1 (□) or allogeneic B6 wild-type (▪) or RANTES-/- (▦) donors as described in Figure 3. In addition, groups of B6D2F1 animals received wild-type B6 bone marrow and RANTES-/- B6 T cells (white lined bar) or RANTES-/- B6 bone marrow and wild-type B6 T cells (gray lined bar). The severity of IPS was subsequently assessed in animals that received transplants by measuring lung histopathology (A) and BALF cellularity (B) 6 weeks after SCT. Data are presented as mean ± SEM and are from 1 experiment (pathology; n = 4 to 9 per group, **P < .01) or from 2 experiments (BAL cellularity; n = 8 to 17 per group, *P < .05. (C) Intracellular RANTES expression in T cells isolated from the lungs 2 weeks after SCT. Mean intensity equals 7.5 ± 0.5 (syn) vs 15.4 ± 2.0 (allo) for CD4+ cells and mean intensity equals 7.3 ± 0.4 (syn) vs 22.5 ± 3.3 (allo) for CD8+ cells. Data presented are from 1 of 2 comparable experiments; solid gray indicates RANTES, thin black line unfilled indicates isotype control; n = 3 per group.
Discussion
The development of acute GVHD and IPS continue to limit the use of allogeneic SCT, particularly when unrelated volunteers are used as stem cell donors. Risk factors for IPS consistently include conditioning with TBI, the presence of acute GVHD, and advanced recipient age.3,4,42-46 The association between GVHD and lung injury in both the acute and chronic settings suggests similar mechanisms of injury may be operative, but a causal relationship between the 2 disorders remains controversial. However, experimental and clinical studies do suggest that synergy between the intensity of SCT conditioning regimens and alloreactive T-cell responses may significantly contribute to the deleterious outcome of IPS after allo-SCT.7,8,47 The pathophysiology of IPS is complex, and both animal and human data support the involvement of inflammatory cytokines and cellular effectors in the evolution of this process.9-17 Although alloreactive Th1/Tc1 lymphocytes and donor monocytes and macrophages have been shown to play a significant role in the evolution of lung injury after allo-SCT,12,16,19-21,40 the mechanisms responsible for leukocyte recruitment to the lung in this setting remain incompletely understood.
In this study, we used an established murine model of IPS to examine the contribution of the CC chemokine RANTES to lung histopathology observed following allo-SCT. Increased RANTES expression in the lung has been previously reported following allo-SCT,18-20 but a mechanistic relationship between RANTES and IPS had not been established. We demonstrate that mRNA and protein levels of RANTES are increased in the lung after allo-SCT. Furthermore, the initial rise in RANTES expression directly correlates with the arrival of donor T cells to the lung. The expression of RANTES remains elevated during the development of IPS and is ultimately associated with the up-regulation of CCR5 and CCR1 mRNA and with the recruitment of additional T cells, monocytes, and macrophages into the pulmonary parenchyma and bronchoalveolar space.
Since the lymphoid and myeloid cell types contributing to IPS can secrete and respond to RANTES, we hypothesized that donor leukocytes may assist in regulating their own recruitment to the lung after transplantation. We found that allo-SCT using RANTES-deficient donor cells results in a significant reduction in the severity of IPS. Importantly, SCT studies wherein wild-type bone marrow cells were admixed with RANTES-deficient T cells and vice versa revealed that RANTES production by donor T cells is particularly important to the subsequent recruitment of leukocytes to the lung after allo-SCT. This observation is supported by our data showing that donor T cells present in the lung by week 2 after allo-SCT actively produce RANTES and by other studies where RANTES production by CD8+ and/or CD4+ T cells directly contributed to the infiltration of cells and the loss of skin and cardiac allografts.48-51
A role for chemokine receptor-to-ligand interactions involving RANTES has been implicated in several models of pulmonary inflammation and also in experimental and clinical allograft rejection.48,49,52-61 In some of these studies, targeting of the biologic effects of RANTES in vivo by using either neutralizing antibodies or intrinsically inactive receptor antagonists resulted in reduced lymphocyte and macrophage infiltration to sites of inflammation.55,56,59-61 Furthermore, specific overexpression of RANTES in the lungs of rats leads to infiltration of cells into the pulmonary parenchyma and bronchoalveolar space, comparable to that seen after allogeneic SCT.62 In the study reported herein, enhanced RANTES expression after allogeneic SCT also was associated with elevations in mRNA levels for CCR1 and CCR5. Similar findings have been observed in the lungs of patients and rodents undergoing lung allograft rejection and were associated with migration of CCR1- and CCR5-positive mononuclear cells in each scenario.61
Despite the protective effect in the lung and an improvement in overall survival, the use of RANTES-deficient donor cells did not significantly reduce hepatic or intestinal inflammation after allo-SCT. These findings are consistent with previous data from several groups, showing that successful inhibition of mechanisms responsible for leukocyte trafficking can reduce lung injury without altering the severity of GVHD in other tissues.17,19,63,64 In particular, chemokine expression patterns differ between GVHD target organs,65 supporting the possibility that individual chemokine receptors may contribute to donor cell migration in a target tissue-specific fashion. Moreover, the contribution of specific chemokine receptor-to-ligand interactions to tissue damage engendered by GVHD is modulated by the intensity of SCT conditioning regiments. For example, the expression of CCR5 on donor T cells was vital for the recruitment of CD8+ T cells to the liver and the Peyer patches after allo-SCT in a nonmyeloablative SCT setting.66,67 Of particular note, migration to the Peyer patches was directly related to the expression of RANTES at that tissue site.66,67 By contrast, the absence of CCR5-/- on donor cells recently has been shown to exacerbate mortality from GVHD under myeloablative conditions.68,69
Our data reveal an important, but not exclusive, role for donor T-cell–derived RANTES in the development of IPS. The residual lung histopathology observed after allo-SCT with RANTES-deficient donor cells may be explained by several factors. RANTES is not solely produced by activated T cells, but also can be expressed by a variety of cell types including fibroblasts, epithelial cells, and endothelial cells.24,26-31,53 Inflammatory cytokines, including TNFα, IFNγ, and IL-1, lead to significant up-regulation of RANTES expression in nonhematopoietic cells,70-73 and these cytokines are significantly elevated following conditioning and allogeneic SCT-to-high-dose chemoradiotherapy results in a proinflammatory milieu that damages host tissues, enhances the allostimulatory capacity of host dendritic cells, and modulates the chemokine environment in host target tissues, including the lung.20,74 Although our mRNA data show that the up-regulation of RANTES in the lung coincided with the arrival of donor T cells, we cannot discount a contribution of host cells to elevated protein levels seen by day 14.
The persistence of lung injury even after in vivo immunoneutralization of RANTES suggests, however, that redundancy in chemokine receptor-to-ligand interactions also contributes to IPS. Mip-1α, Mip-1β, and MCP-2 all share the chemokine receptors CCR1 and CCR5 with RANTES.34,75 Although specific roles for Mip-1β and MCP-2 in the development of IPS have yet to be determined, the contribution of Mip-1α has been explored. A role for donor-derived Mip-1α in the recruitment of leukocytes to the lung after SCT under nonmyeloablative conditions was noted in one report.76 Subsequent work by the same group in a myeloablative model suggested, however, that the use of Mip-1α-/- mice as allo-SCT donors exacerbated, rather than reduced, early lung injury.77 Thus, these findings indirectly support our data that receptor-to-ligand interactions involving RANTES may have a dominant role in the evolution of IPS. In addition, we have recently demonstrated specific roles for both CCR2-to-MCP-1 and CXCR3-to-Mig/IP-10 interactions with respect to the recruitment of donor macrophages (CCR2) and T lymphocytes (CXCR3) to the lung after allo-SCT.19,21 Collectively, these observations suggest that complex and redundant chemokine receptor-to-ligand interactions contribute to the development of IPS. Although targeting individual pathways may not completely abrogate pulmonary histopathology after allo-SCT, such strategies can reveal mechanistic insights to leukocyte recruitment during IPS as observed in animal models of bronchiolitis obliterans and cardiac rejection.57,58,61,78,79
We hypothesize that donor T cells serve as both effectors and facilitators of lung injury after allo-SCT. Chemokine receptor-to-ligand interactions involving CXCR3 likely contribute to the initial recruitment of alloreactive T cells to the lung.21,63,80 Once present, these cells may (1) directly contribute to parenchymal injury via cytolytic pathways81 and via the secretion of cytokines including IFNγ, TNFα, and RANTES, (2) alter the local chemokine milieu,20,63 and (3) promote self-recruitment along with the recruitment of other accessory cells to the lung during the development of IPS. The subsequent influx of leukocytes and release of soluble mediators further modifies the local inflammatory environment and propagates pulmonary injury and dysfunction. This hypothesis would therefore suggest that novel strategies targeting the regulation of effector cell recruitment to the lung (or other GVHD target tissues) might serve as effective adjuncts to standard therapy intended to prevent or treat these serious complications of allo-SCT.
Prepublished online as Blood First Edition Paper, November 16, 2004; DOI 10.1182/blood-2004-08-3320.
An Inside Blood analysis of this article appears in the front of this issue.
Supported by National Institutes of Health grants 5K12HD028820-12, R01 HL072258-02, R01 HL55162-05, R01 CA102052-02, and the Walther Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Dr Hildebrandt is a Deutsche Krebshilfe e.V. Scholar. Dr Cooke is an Amy Strelzer Manasevit Scholar of the National Marrow Donor Program, a Fellow of the Robert Wood Johnson Foundation, Harold Amos Medical Faculty Development Program, and the Recipient of a Translational Research Award from the Leukemia and Lymphoma Society.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal