Abstract
Tumor dormancy is a phenomenon where small numbers of tumor cells persist in the host for months or years. We previously showed in the DA1-3b/C3H mouse model of acute myeloid leukemia that dormant tumor cells resist cytotoxic T-lymphocyte (CTL)–mediated killing because they overexpress B7-H1. Here, we vaccinated mice with DA1-3b cells transduced with CXCL10. Vaccinated mice developed a strong systemic immunity that led to the cure of established leukemia without persistence of dormant tumor cells. In vivo depletion of natural killer (NK) cells from the mice abrogated the protective effect of the vaccine. Long-term persistent leukemic cells resist CTL-mediated lysis but were killed by NK cells from mice vaccinated with DA1-3b/CXCL10. These NK cells expressed B7-H1. Recombinant CXCL10, CXCL9, CXCL11, and CXCL12 chemokines induced expression of B7-H1 on mouse and human NK cells in vitro. Mouse and human B7-H1+ NK cells induced proliferation of T cells and production of interferon γ and tumor necrosis factor α in vitro, and in vivo blocking of B7-H1 inhibited the protective effect of vaccination. Thus, CXCL10 induces antileukemic immunity, at least partially by stimulating NK cells to express B7-H1+. This antitumor effect is in contrast to the effect of B7-H1 when expressed on tumor cells because it stops cytotoxic lymphocytes from killing those tumor cells.
Introduction
Tumor dormancy is a condition in which tumor cells persist in the host for a long time but do not proliferate.1 It is a common phenomenon in cancer, and eradication of tumor dormant cells is an important goal toward the definitive cure of malignancies.
We previously reported in the DA1-3b/C3H mouse model of acute myeloid leukemia (AML) that vaccination of mice with leukemia cells that had been transduced with CD154, granulocyte-macrophage colony-stimulating factor (GM-CSF), or interleukin 12 (IL-12) genes led to significant protection against leukemia, and generated specific cytotoxic T lymphocytes (CTLs) against leukemic cells.2-4 Despite these responses, we detected several hundred persistent leukemic cells in some long-term surviving mice. Persistent leukemic cells were more resistant to specific CTL-mediated killing because they expressed more B7-H1 and B7.1, and expression was proportional to the time they had persisted in the host.4
Eradication of tumor dormant cells in AML would require the generation of cytotoxic cells that cannot be inhibited by B7-H1 or B7.1. We observed in previous experiments that minimal residual disease (MRD) was less common in the spleen of mice vaccinated with irradiated IL-12–transduced cells than those vaccinated with cells transduced with other genes, like CD154.4 CXCL10 (also called interferon-inducible protein 10 [IP-10]) is an essential mediator of the antitumor effect of IL-12.5-7 CXCL10 is a CXC chemokine structurally and functionally related to monokine induced by interferon γ (IFN-γ; Mig, CXCL9) and IFN-inducible T-cell α chemoattractant (I-TAC, CXCL11).8-10 CXCL10, like CXCL9 and CXCL11, acts though the chemokine receptor CXCR3. These chemokines attract activated T cells and natural killer (NK) cells.11-14 CXCL10-deficient mice also had a defect in effector T-cell generation, indicating that CXCL10 might also be essential to T-cell adaptive immunity.15 We therefore used the DA1-3b mouse model of AML to investigate if CXCL10 could induce an efficient antileukemic immune response.
Material and methods
Mouse AML model
Female C3H/HeJ mice, 8 to 10 weeks old, were obtained from Charles River Laboratories (Lyon, France). The leukemic DA1-3b BCR/ABL-expressing cell line has been described previously.2,16 Mice injected intraperitoneally with 104 to 106 DA1-3b cells developed systemic lethal leukemia in 3 to 4 weeks, with massive infiltration of bone marrow, spleen, and peripheral blood.
Transfection of DA1-3b cells
pGT-IL12, pGT-CXCL10, and pGT-Zeo were purchased from Invivogen (Toulouse, France). DA1-3b cells were electroporated at 1500 μF and 250 V and further selected with Zeomycin (Invitrogen). Large batches of selected transduced cells were frozen until use. Expression of IL-12 and CXCL10 was assessed by enzyme-linked immunosorbent assay (ELISA; IL-2, OptEIA kit; CXCL10, rat IgG1 anti-mIP-10; PharMingen, San Diego, CA).
Immunization of mice and quantification of residual disease
Groups of 10 C3H/Hej mice were injected intradermally with irradiated (100 Gy from a 137C source) IL-12– or CXCL10-transduced cells (termed DA1-3b/IL12 and DA1-3b/CXCL10, respectively). DA1-3b cells transfected with the pGT-Zeo empty plasmid (DA1-3b/Zeo) were used as controls. Cells were diluted in 200 μL phosphate-buffered saline (PBS) before injection. Each mouse received 3 injections, a week apart, and immunity was challenged a week after the last injection by intraperitoneal injection of 104 to 106 leukemic DA1-3b cells. For therapeutic experiments, mice were first injected intraperitoneally with 104 to 106 leukemic DA1-3b cells, and then 3 days later received the first of 3 weekly intradermal injections of 106 irradiated DA1-3b/CXCL10, DA1-3b/IL12, or DA1-3b/Zeo cells. Experiments were repeated at least 3 times. In mice that survived 1 year, persisting leukemic cells were quantified by real-time quantitative polymerase chain reaction (RQ-PCR) on gDNA extracted from all organs.4
In vivo depletion and blocking experiments
Hybridoma cell lines GK-1.5 (rat antimouse CD4+) and 53-6.72 (rat antimouse CD8+) from American Type Culture Collection (Rockville, MD), and antimouse IFN-γ (clone XMG1.2), antimouse tumor necrosis factor α (TNF-α; clone MP3-XT3; PharMingen), and antimouse B7-H1 (clone MIH5; Clinisciences, Montrouge, France) were used as a source of monoclonal antibodies (mAbs). Groups of 11 C3H/Hej mice were vaccinated on 3 occasions, 1 week apart, by intradermal injection of 106 transduced DA1-3b cells, or DA1-3b/Zeo cells as controls, irradiated at 100 Gy. For depletion, mice were then injected intraperitoneally on 3 consecutive days with an anti-CD4 or anti-CD8 (500 μg/injection) and 1 week later antileukemic immunity was challenged by intraperitoneal injection of 104 wild-type DA1-3b cells. Efficacy of depletion was checked by flow cytometry on splenocytes from one mouse per group. For depletion of NK cells, mice were injected intraperitoneally with 500 μg antiasialo GM1 (Wako, Osaka, Japan) on each of 3 consecutive days and weekly after the challenge. To block IFN-γ and TNF-α, mice were injected intraperitoneally on each of 3 consecutive days with the blocking antibody or with a control isotype (500 μg/injection). To block B7-H1, mice were first injected with 106 irradiated transduced DA1-3b cells. Each mouse received 3 intraperitoneal injections, a week apart and 2 days after the last injection, of 500 μg anti-B7-H1 (clone MIH5), or the isotype control mAb (rat IgG2a, λ, Clinisciences).4,17,18
Time-course analysis of immune cellular infiltrates with matrigel
Recruitment of immune cells induced by transduced leukemic cells was analyzed using a matrigel technique adapted from Kowalczyk et al.19 The matrigels were introduced by intradermal injection of a mixture of 0.5 mL liquefied matrigel (BD Biosciences, San Jose, CA) and 5 × 106 irradiated DA1-3b/Zeo, DA1-3b/IL12, or DA1-3b/CXCL10 cells diluted in 200 μL PBS. Ten mice were killed at day 1, 2, 3, 5, 7, 10, and 15, and matrigels from the site of vaccination were harvested by skin excision. Matrigels were liquefied in 0.3 mL PBS at 4°C overnight. Isolated cells were analyzed by flow cytometry with mAbs against CD8-fluorescein isothiocyanate (FITC; clone 53-3.1), CD3-phycoerythrin (PE; clone 17A2), CD4-PE (clone H129.19), pan NK-FITC (clone DX5), B7.1-FITC (clone 1G10), B7.2-PE (clone GL1), and CD154-PE (clone MR1) from PharMingen; PD1-FITC (clone J43), FasL-PE (clone MFL3), B7-H1-PE (clone MIH5), OX40L-PE (clone RM134L), OX40-FITC (clone ACT-35), 4-1BB-PE (clone 17B5), and 4-1BBL-PE (clone TKS-1) from Clinisciences; B220-PE (clone RA3-6B2), CD19-FITC (clone 1D3), CD11c-PE (clone HL3), and CD11b (clone M1/70) from PharMingen.
Assay for CTLs and NK cells
Mice were injected subcutaneously with 106 irradiated DA1-3b cells transduced with pGT-IL12, pGT-CXCL10, or pGT-Zeo. Spleens were removed from these mice and from naive mice, and lymphocytes were isolated with a Miltenyi-Biotec separation column and anti-CD90 (THY1.2) mAb, according to the manufacturer's recommendations (Miltenyi Biotec, Bergisch Gladbach, Germany). CD90+ cells were cocultured with irradiated DA1-3b cells and 20 IU murine IL-2/mL (PreproTech, Rocky Hill, NJ) at 5 mL/well in a 6-well tissue culture plate. For the CTL assay, CD8+ cells were isolated with the CD8a+ T-cell isolation kit (Miltenyi Biotec). CTL activity was measured after 15 days of coculture, using the Cytotox 96 nonradioactive assay (Promega, Madison, WI) with cultured cells from vaccinated mice as effectors, and DA1-3b cells as targets, mixed at various ratios. Major histocompatibility complex (MHC) class I restricted CTL lysis of DA1-3b cells was verified using anti-MHC I and anti-MHC II blocking antibodies.4 Absence of NK cell-mediated cytotoxicity was verified with the YAC-1 cell line. Specificity of CD8+ CTL lysis against DA1-3b cells was controlled with the EL4 cell line.
For NK cell isolation, mice were injected intradermally with 106 irradiated DA1-3b cells transduced with pGT-IL12, pGT-CXCL10, or pGT-Zeo, and injections were repeated weekly 3 times. One week later mice were humanely killed and the spleen was removed. NK cells were isolated using an NK cell isolation kit according to the manufacturer's recommendations (Miltenyi Biotec). Purity of NK cell samples was analyzed by flow cytometry using anti-B220 and anti-CD122 mAbs (PharMingen). NK cell cytotoxicity was analyzed at the day of isolation using the Cytotox 96 nonradioactive cytotoxicity assay (Promega) by mixing NK cells isolated from the spleen as effectors and DA1-3b or YAC-1 cells as targets at various ratios. To inhibit perforin-mediated cytotoxicity, effector cells were incubated for 2 hours in 100 nM concanamycin A (CMA; Sigma, St Louis, MO).
In vitro treatment of mouse and human NK cells with chemokines and cytokines
Purified NK cells from naive mice were incubated with recombinant IL-2 (200 IU/mL), IL-3 (200 IU/mL), IL-4 (10 ng/mL), IL-5 (30 IU/mL), IL-7 (50 ng/mL), IL-10 (100 IU/mL), IL-12 (20 IU/mL), IL-13 (50 ng/mL), IL-15 (100 ng/mL), IL-18 (50 ng/mL), GM-CSF (100 ng/mL), IFN-γ (200 IU/mL), TNF-α (500 IU/mL), CXCL9 (100 ng/mL), CXCL10 (100 ng/mL), CXCL11 (200 ng/mL), CXCL12 (40 ng/mL), CCL3 (100 ng/mL), CCL5 (100 ng/mL), or CCL11 (100 ng/mL). Flow cytometry analysis of B7-H1 expression was performed after 6 and 24 hours of incubation. Human NK cells were isolated from 5 healthy volunteers who had provided their informed consent. The cell isolation was performed using an NK cell isolation kit (Miltenyi Biotec), following which cells were incubated with recombinant IL-2, IL-3, IL-4, GM-CSF, IL-12, IFN-γ, TNF-α, CXCL9, CXCL10, CXCL11, CXCL12, CCL3, CCL5, and CCL11 at the same doses used in the mouse experiments. Human CD56brightCD16dim and CD56dimCD16bright NK cells subpopulations were purified by cell sorting on a EPICS ALTRA sorter (Beckman Coulter, Marseille, France) based on mean fluorescence intensity (MFI) expression, assessed using human CD56 (clone B159; PharMingen) and CD16 mAb, incubated in vitro with recombinant CXCL10, and then analyzed for B7-H1 expression after 6 hours and 24 hours.
Cocultures of NK cells with CFSE-labeled CD4+ and CD8+ T cells
Mouse or human CD8+ or CD4+ T cells were washed in PBS, suspended at 106 cells/mL, and labeled with 10 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes Europe, Leiden, The Netherlands) for 10 minutes at 37°C. Cells were again washed twice before addition to cocultures with NK cells at a 1:1 ratio and with 200 IU/mL IL-2. At the indicated times CFSE-labeled cells were collected, washed twice in PBS/1% bovine serum albumin (BSA), and fixed in PBS/1% paraformaldehyde. For 2-color fluorescence studies, CFSE-labeled cells were incubated with saturating concentrations of directly PE-conjugated antimouse CD4 or CD8 mAbs for 30 minutes at 4°C, washed twice, and subjected to flow cytometry analysis. Unlabeled and labeled CD8+ and CD4+ T cells, fixed immediately after CFSE labeling, were used as negative and positive internal controls for staining. Expression of mouse IFN-γ, TNF-α, IL-4, and IL-10, and human IFN-γ, TNF-α, IL-4, and IL-10 were assessed by intracellular staining with appropriate antibodies (PharMingen). Mouse and human B7-H1 were blocked with antimouse B7-H1 (clone MIH5) and antihuman B7-H1 (clone MIH1) antibodies, respectively. Mouse and human programmed death 1 (PD-1) were blocked with antimouse PD-1 (clone J43) and antihuman PD-1 (clone J116) antibodies, respectively.
Statistical analysis
Statistical analysis was performed using the Sigma Stat 3.0 software (SPSS Science, Chicago, IL).
Results
Prophylactic and therapeutic immunity induced by DA1-3b/CXCL10 cells
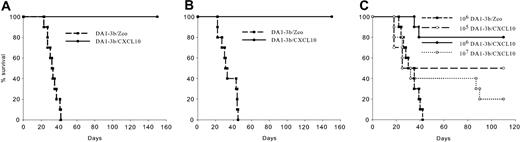
DA1-3b cells transfected with pGT-CXCL10 (DA1-3b/CXCL10) or pGT-IL12 plasmid (DA1-3b/IL12) produced 3600 ± 20 pg CXCL10/106 cells/mL/24 h and 2100 ± 55 pg IL12/106 cells/ml/24 h, respectively. In prophylactic experiments, mice vaccinated with irradiated DA1-3b/CXCL10 cells showed better survival than those vaccinated with DA1-3b/IL12 or control DA1-3b/Zeo cells (Figure 1A-1B), even when challenged with 106 wild-type DA1-3b cells (Figure 1C).
Irradiated DA1-3b/CXCL10 leukemic cells induce prophylactic and therapeutic immunity. (A-C) Vaccine experiments. C3H/Hej mice were vaccinated with 106 irradiated DA1-3b leukemic cells that had been transduced with CXCL10 (DA1-3b/CXCL10; •), IL-12 (DA1-3b/IL-12; ▿), or control empty plasmid (DA1-3b/Zeo; ▪), and the mice were subsequently challenged with (A) 104, (B) 105, or (C) 106 wild-type DA1-3b cells. (D-F) Therapeutic experiments. C3H/Hej mice were injected with (D) 104, (E) 105,or (F) 106 wild-type DA1-3b/cells. Three days later mice were given injections of irradiated DA1-3b/CXCL10, DA1-3b/IL12, or DA1-3b/Zeo leukemic cells. Data represent 30 mice/group in 3 separate experiments.
Irradiated DA1-3b/CXCL10 leukemic cells induce prophylactic and therapeutic immunity. (A-C) Vaccine experiments. C3H/Hej mice were vaccinated with 106 irradiated DA1-3b leukemic cells that had been transduced with CXCL10 (DA1-3b/CXCL10; •), IL-12 (DA1-3b/IL-12; ▿), or control empty plasmid (DA1-3b/Zeo; ▪), and the mice were subsequently challenged with (A) 104, (B) 105, or (C) 106 wild-type DA1-3b cells. (D-F) Therapeutic experiments. C3H/Hej mice were injected with (D) 104, (E) 105,or (F) 106 wild-type DA1-3b/cells. Three days later mice were given injections of irradiated DA1-3b/CXCL10, DA1-3b/IL12, or DA1-3b/Zeo leukemic cells. Data represent 30 mice/group in 3 separate experiments.
Next, we examined the therapeutic effect of DA1-3b/CXCL10 cells on established leukemia. Seventy-nine percent of mice injected with 104 DA1-3b cells survived when given irradiated DA1-3b/CXCL10 cells, but only 38% of mice that received irradiated DA1-3b/IL12 cells did so (Figure 1D). Irradiated DA1-3b/CXCL10 cells increased long-term survivals when 105 DA1-3b cells were injected (Figure 1E) and delayed death of mice injected with 106 cells (P < .001, log-rank test; Figure 1F). Mice vaccinated with DA1-3b/CXCL10 cells that survived to the first injection of 104 DA1-3b cells were rechallenged 3 months later with 104 or 105 leukemic cells. As shown in Figure 2A-2B, all mice were still protected from leukemia, demonstrating a long-term immunologic memory. The immunity induced by DA1-3b/CXCL10 cells was dose-dependent. Mice vaccinated with 106 DA1-3b/CXCL10 cells were significantly better protected than those injected with only 105 cells (P = .037, log-rank test; Figure 2C). However, vaccination with 107 cells resulted in a lower survival rate (P = .005, log-rank test).
Dose-dependent vaccine effect, and induction of memory immunity, by vaccination with irradiated DA1-3b leukemic cells transduced with the gene for CXCL10. (A) C3H/Hej mice were vaccinated with 106 irradiated DA1-3b/CXCL10 cells and then challenged by 104 wild-type DA1-3b cells (•). Mice that survived this were rechallenged 3 months later with the same dose of leukemic cells. A control group of 10 naive mice was vaccinated with DA1-3b/Zeo cells and challenged at the same time (▪). (B) Same procedure as in panel A but mice were rechallenged with 105 wild-type DA1-3b cells. (C) C3H/Hej mice were vaccinated with 105 (○), 106 (•), or 107 irradiated DA1-3b/CXCL10 cells ( ), and the mice were subsequently challenged with 105 wild-type DA1-3b cells. ▪ represents control cells.
), and the mice were subsequently challenged with 105 wild-type DA1-3b cells. ▪ represents control cells.
Dose-dependent vaccine effect, and induction of memory immunity, by vaccination with irradiated DA1-3b leukemic cells transduced with the gene for CXCL10. (A) C3H/Hej mice were vaccinated with 106 irradiated DA1-3b/CXCL10 cells and then challenged by 104 wild-type DA1-3b cells (•). Mice that survived this were rechallenged 3 months later with the same dose of leukemic cells. A control group of 10 naive mice was vaccinated with DA1-3b/Zeo cells and challenged at the same time (▪). (B) Same procedure as in panel A but mice were rechallenged with 105 wild-type DA1-3b cells. (C) C3H/Hej mice were vaccinated with 105 (○), 106 (•), or 107 irradiated DA1-3b/CXCL10 cells ( ), and the mice were subsequently challenged with 105 wild-type DA1-3b cells. ▪ represents control cells.
), and the mice were subsequently challenged with 105 wild-type DA1-3b cells. ▪ represents control cells.
To investigate if CXCL10 could also eradicate persistent leukemic cells, we vaccinated mice with irradiated DA1-3b/CXCL10, DA1-3b/IL12, DA1-3b/CD154, or DA1-3b/Zeo cells, challenged immunity with live DA1-3b cells, and then extracted DNA of all organs of every mouse that survived 1 year. Leukemic cells were found in the spleen and liver of mice vaccinated with DA1-3b/IL12, DA1-3b/CD154, and DA1-3/Zeo, as previously described.4 However, no DA1-3b cells could be found in 65 mice from the DA1-3b/CXCL10 vaccine group (Table 1) and the difference with the DA1-3b/IL12 group was significant (P < .001, χ2 test).
Effect of cell vaccines on development of MRD in mice
Vaccine . | No. of mice . | No. of MRD+ mice (%) . |
|---|---|---|
| DA1-3b/CXCL10 | 65 | 0 (0) |
| BA1-3b/Zeo | 8 | 6 (62.5) |
| DA1-3b/IL12 | 47 | 9 (19) |
| DA1-3b/CD150 | 26 | 22 (85) |
Vaccine . | No. of mice . | No. of MRD+ mice (%) . |
|---|---|---|
| DA1-3b/CXCL10 | 65 | 0 (0) |
| BA1-3b/Zeo | 8 | 6 (62.5) |
| DA1-3b/IL12 | 47 | 9 (19) |
| DA1-3b/CD150 | 26 | 22 (85) |
Mice were vaccinated with irradiated DA1-3b cells transduced with vector containing IL-12 (DA1-3b/IL12), CXCL10 (DA1-3b/CXCL10), CD154 (DA1-3b/CD154), or control empty vector (DA1-3b/Zeo) and then inoculated with 104 live DA1-3b cells. After 1 year, surviving mice were tested by real-time PCR for the presence of MRD.
In vivo depletion and blocking experiments
To identify the effectors of the antileukemic immunity induced by CXCL10, we vaccinated mice with irradiated DA1-3b/CXCL10 or with control DA1-3b/Zeo cells, depleted them with anti-CD4, anti-CD8, or anti-NK antibodies or control isotypes, and challenged immunity by injection of 105 wild-type DA1-3b cells. As shown in Figure 3A, the protective effect of CXCL10 was completely dependent on NK cells. Depletion of CD4 or CD8 T cells partially abolished the vaccine effect (Figure 3B-C).
Survival of vaccinated mice after depletion of effector cells or blocking IFN-γ or TNF-α. C3H/Hej mice were vaccinated with 106 irradiated DA1-3b leukemic cells that had been transduced with CXCL10 (DA1-3b/CXCL10) or control empty plasmid (DA1-3b/Zeo) and injected with (A) anti-NK, (B) anti-CD4, (C) anti-CD8, (D) anti-IFN-γ, (E) anti-TNF-α, or control isotype-depleting antibodies. Mice were subsequently challenged with 105 wild-type DA1-3b cells.
Survival of vaccinated mice after depletion of effector cells or blocking IFN-γ or TNF-α. C3H/Hej mice were vaccinated with 106 irradiated DA1-3b leukemic cells that had been transduced with CXCL10 (DA1-3b/CXCL10) or control empty plasmid (DA1-3b/Zeo) and injected with (A) anti-NK, (B) anti-CD4, (C) anti-CD8, (D) anti-IFN-γ, (E) anti-TNF-α, or control isotype-depleting antibodies. Mice were subsequently challenged with 105 wild-type DA1-3b cells.
We also injected blocking antibodies or control isotypes against IFN-γ and TNF-α. The CXCL10 vaccine effect was completely dependent on IFN-γ but independent of TNF-α (Figure 3D-E).
Time course of recruitment of immune cells at the vaccine site
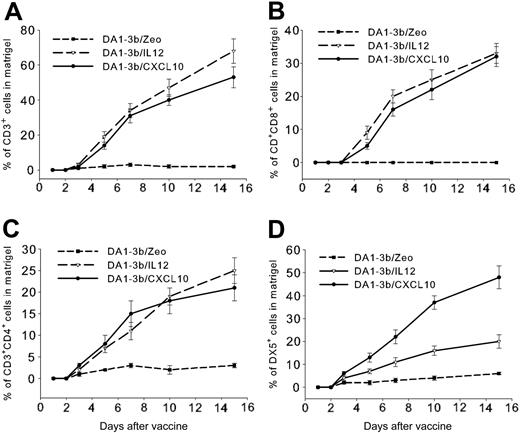
To investigate which cells the leukemia vaccine attracted, we diluted 106 irradiated DA1-3b/IP10, DA1-3b/IL12, or control DA1-3b/Zeo cells in matrigel and injected 100 μL intradermally into mice. Gels were excised at different times and flow cytometry analysis of cells in the resolubilized matrigel revealed a progressive infiltration by CD3+, CD3+CD4+, and CD3+CD8+ T cells induced in similar proportions by DA1-3b/IL12 or DA1-3b/CXCL10 cells (Figure 4A-C). However, DA1-3b/CXCL10 cells induced significantly higher infiltration of the vaccine site by NK cells than DA1-3b/IL12 cells (Figure 4D). No B220+CD19+B cells could be detected in the infiltrates. CD11c+CD11b+ dendritic cells were detected only 15 days after injection and represented 11% ± 3%, 12% ± 3%, and 16% ± 4% of cells in infiltrates from DA1-3b/Zeo, DA1-3b/IL12, and DA1-3b/CXCL10 matrigels, respectively. These slight differences were statistically significant (P = .021, Student t test).
Time course of immune cell infiltration at the vaccine site. Seventy C3H/Hej mice were injected intradermally with 500 μL liquid matrigel with 106 irradiated DA1-3b/CXCL10 (•), DA1-3b/IL-12 (▿), or control DA1-3b/Zeo leukemic cells (▪). Gels were excised at selected times and infiltrating cells were analyzed by flow cytometry for percentage of cells that were (A) CD3+ T cells, (B) CD3+CD4+ T cells, (C) CD3+CD8+ T cells, and (D) DX5+ NK cells. Data represent mean and SD from 10 mice analyzed in triplicate.
Time course of immune cell infiltration at the vaccine site. Seventy C3H/Hej mice were injected intradermally with 500 μL liquid matrigel with 106 irradiated DA1-3b/CXCL10 (•), DA1-3b/IL-12 (▿), or control DA1-3b/Zeo leukemic cells (▪). Gels were excised at selected times and infiltrating cells were analyzed by flow cytometry for percentage of cells that were (A) CD3+ T cells, (B) CD3+CD4+ T cells, (C) CD3+CD8+ T cells, and (D) DX5+ NK cells. Data represent mean and SD from 10 mice analyzed in triplicate.
Vaccine cells induced also a significant modification of the proportion of immune cells distant from the vaccine site. Flow cytometry analysis of the spleen of vaccinated animals showed higher proportions of CD3+ (P = .004) and CD3+CD4+ (P = .014) T cells in mice vaccinated with DA1-3b/IL12 cells and higher proportion of NK cells (P = .009) in mice vaccinated with DA1-3b/CXCL10 cells (Table 2).
T and NK cells in the spleen of vaccinated mice
. | Percent of splenocytes (SD) . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Vaccine . | CD3 . | CD4 . | CD8 . | NK . | |||
| DA1-3b/CXCL10 | 57 (7) | 21 (4) | 34 (6) | 38 (5) | |||
| DA1-3b/IL12 | 76 (8) | 28 (3) | 39 (5) | 29 (3) | |||
| DA1-3b/Zeo | 11 (2) | 5 (2) | 5 (2) | 24 (3) | |||
. | Percent of splenocytes (SD) . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Vaccine . | CD3 . | CD4 . | CD8 . | NK . | |||
| DA1-3b/CXCL10 | 57 (7) | 21 (4) | 34 (6) | 38 (5) | |||
| DA1-3b/IL12 | 76 (8) | 28 (3) | 39 (5) | 29 (3) | |||
| DA1-3b/Zeo | 11 (2) | 5 (2) | 5 (2) | 24 (3) | |||
Mice were vaccinated with irradiated DA1-3b cells transduced with vector containing IL-12 (DA1-3b/IL 12), CXCL10 (DA1-3b/CXCL10), or control empty vector (DA1-3b/Zeo), and spleen cells were analyzed by flow cytometry.
CD8+ CTL and NK-cell cytotoxicity against leukemic cells and persistent leukemic cells
To evaluate the relative role of CD8+ CTL and NK cells as effectors of the antileukemic immune response and in the control of dormant tumor cells, we isolated T cells and NK cells from mice vaccinated with DA1-3b/CXCL10, DA1-3b/IL12, control DA1-3b cells or naive mice as previously described.4 CD8+ CTLs isolated from mice vaccinated with DA1-3b/CXCL10 showed lower lytic activity against leukemic DA1-3b cells than those from the DA1-3b/IL12 group (Figure 5A). As previously reported, persistent leukemic cells isolated 1, 3, and 12 months of survival after challenge from the spleen of DA1-3b/IL12–vaccinated mice showed resistance to CD8+ CTL-specific lysis proportional to the time they spent in the host (Figure 5B).4 NK cells isolated from DA1-3b/CXCL10–vaccinated mice showed higher lytic activity against DA1-3b cells than did NK cells from DA1-3b/IL12–vaccinated mice (Figure 5C). EL4 thymoma cells, which have been reported to be relatively resistant to NK-mediated lysis, were killed by NK cells isolated from DA1-3b/CXCL10–vaccinated mice in similar proportion as DA1-3b cells and positive control YAC-1 cells (Figure 5C).24 NK cells from DA1-3b/CXCL10–vaccinated mice killed persistent leukemic cells with the same efficacy whatever time these cells have spent in the host (Figure 5D). NK cell cytotoxicity was partially inhibited by CMA, indicating that the cytolytic effect was at least partially mediated by the perforin/granzyme pathway (Figure 5D). We previously showed that resistance of dormant tumor cells to CD8+ CTL lysis was mediated by overexpression of B7-H1 and B7.1.4 NK cells from vaccinated mice express PD-1, the only known receptor for B7-H1, CD28, and CTLA-4 (data not shown). To explore a possible role for B7-H1/PD-1 and B7.1/CD28 or CTL antigen 4 (CTLA4) in NK/persistent leukemic cells interaction, we added antibodies against B7-H1 or B7.1, or control isotypes, to the NK cell cytotoxicity assay. No inhibitory or activating effect could be observed (Figure 5D).
Sensitivity of persistent leukemic cells to NK- and CTL-mediated killing. (A) Sensitivity to CTL-mediated killing of DA1-3b leukemic cells. Mice were vaccinated with leukemic cells that had been transduced with CXCL10 (DA1-3b/CXCL10), IL-12 (DA1-3b/IL-12), or with control empty plasmid (DA1-3b/Zeo), and CTLs were isolated. (B) Mice were vaccinated with irradiated DA1-3b/IL12 cells, following which CTLs were isolated and tested for their ability to kill leukemic cells that had persisted in other animals for 1 month (DA1-3b/d35), 3 months (DA1-3b/d90), and 1 year (DA1-3b/d365). (C) Sensitivity to NK cell-mediated killing of DA1-3b leukemic cells, YAC-1, and EL4 cells. NK cells were isolated from mice vaccinated with DA1-3b/CXCL10, DA1-3b/IL12, or DA1-3b/Zeo. (D) NK cells were isolated from mice vaccinated with irradiated DA1-3b/CXCL10 cells and tested for their ability to kill leukemic cells that had persisted in other animals for 1 month (DA1-3b/d35), 3 months (DA1-3b/d90), and 1 year (DA1-3b/d365), in the presence of anti-B7H1–blocking antibodies, anti-B7.1–blocking antibodies, or control isotypes. CMA was added to DA1-3b cells to block the perforin/granzyme cytolytic pathway (CMA + DA1-3b). (E) Flow cytometry analysis of B7-H1 expression in NK cells isolated from immune infiltrates in matrigel at the vaccine site. The bold line represents anti-B7-H1 labeling, and the thin line represents the control isotype. All experiments were performed in quadruplicate and repeated at least 3 times. Data represent mean and SD.
Sensitivity of persistent leukemic cells to NK- and CTL-mediated killing. (A) Sensitivity to CTL-mediated killing of DA1-3b leukemic cells. Mice were vaccinated with leukemic cells that had been transduced with CXCL10 (DA1-3b/CXCL10), IL-12 (DA1-3b/IL-12), or with control empty plasmid (DA1-3b/Zeo), and CTLs were isolated. (B) Mice were vaccinated with irradiated DA1-3b/IL12 cells, following which CTLs were isolated and tested for their ability to kill leukemic cells that had persisted in other animals for 1 month (DA1-3b/d35), 3 months (DA1-3b/d90), and 1 year (DA1-3b/d365). (C) Sensitivity to NK cell-mediated killing of DA1-3b leukemic cells, YAC-1, and EL4 cells. NK cells were isolated from mice vaccinated with DA1-3b/CXCL10, DA1-3b/IL12, or DA1-3b/Zeo. (D) NK cells were isolated from mice vaccinated with irradiated DA1-3b/CXCL10 cells and tested for their ability to kill leukemic cells that had persisted in other animals for 1 month (DA1-3b/d35), 3 months (DA1-3b/d90), and 1 year (DA1-3b/d365), in the presence of anti-B7H1–blocking antibodies, anti-B7.1–blocking antibodies, or control isotypes. CMA was added to DA1-3b cells to block the perforin/granzyme cytolytic pathway (CMA + DA1-3b). (E) Flow cytometry analysis of B7-H1 expression in NK cells isolated from immune infiltrates in matrigel at the vaccine site. The bold line represents anti-B7-H1 labeling, and the thin line represents the control isotype. All experiments were performed in quadruplicate and repeated at least 3 times. Data represent mean and SD.
Expression of B7-H1 on NK cells
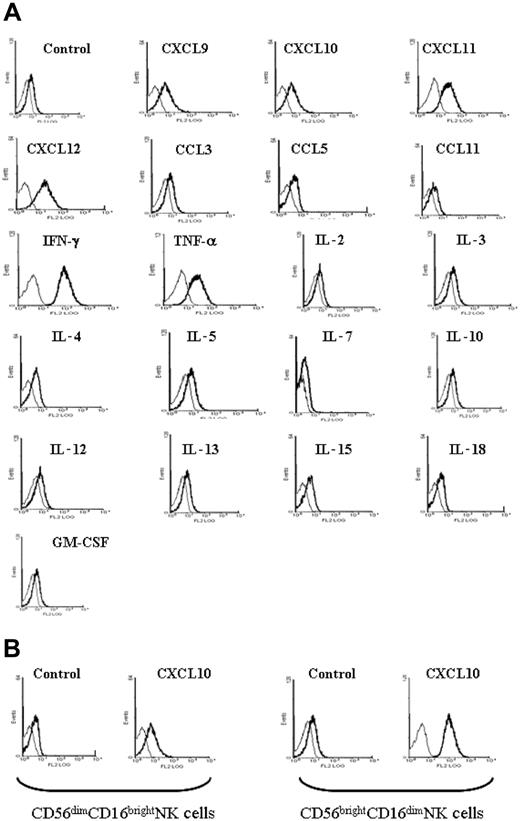
To analyze how NK cells could contribute to the antileukemic immunity observed in mice vaccinated with DA1-3b/CXCL10 cells, we analyzed the expression of several molecules that stimulate T cells. No variation of expression of B7.1, B7.2, 4-1BB, 4-1BBL, OX40, OX40L, CD154, Fas, FasL, Trail, PD1, and B7-H2 was seen between NK cells from matrigels from the different groups of vaccinated mice (see “Time course of recruitment of immune cells at the vaccine site”) or from spleen (data not shown). The LY49D-activating NK receptor was expressed at the same level in NK cells from mice vaccinated with DA1-3b/CXCL10, DA1-3b/IL12, or DA1-3b/Zeo cells. LY49A, LY49I, LY49H, and LY49F were not expressed. However, B7-H1 expression was detected in NK cells from spleen, matrigel infiltrates, and blood from mice vaccinated with DA1-3b/CXCL10 (Figure 5E). To verify if this induction of B7-H1 expression was specific to CXCL10, we incubated NK cells from naive mice with recombinant CXCL10 or with the structurally related chemokines CXCL9 and CXCL11. We observed the same effect in all (Figure 6A). CXCL12 (stromal-derived factor 1α [SDF-1α]), IFN-γ, and TNF-α were also effective. However, the 3 CC chemokines tested, CCL11 (eotaxin), CCL5 (RANTES), and CCL3 (macrophage inflammatory protein 1α [MIP-1α]) showed no effect. IL-3, IL-4, IL-6, IL-10, IL-13, IL-15, IL-18, and GM-CSF showed no or little effect. Human NK cells, incubated with recombinant human CXCL10, CXCL9, CXCL10, CXCL11, CXCL12, IFN-γ, or TNF-α, showed increased B7-H1 expression, but IL-2, IL-3, IL-4, GM-CSF, IL-12, CCL3, CCL5, and CCL11 had no effect. The purified CD56brightCD16dim NK cell subpopulation expressed higher levels of B7-H1 than the CD56dimCD16bright NK cell subset after incubation with CXCL10 (Figure 6B).
Cytokine stimulation of B7-H1 expression in mouse and human NK cells. Cells isolated from (A) naive mice and (B) humans were incubated in vitro with various recombinant chemokines and cytokines. The bold line represents anti-B7-H1 labeling and the thin line represents the control isotype.
Cytokine stimulation of B7-H1 expression in mouse and human NK cells. Cells isolated from (A) naive mice and (B) humans were incubated in vitro with various recombinant chemokines and cytokines. The bold line represents anti-B7-H1 labeling and the thin line represents the control isotype.
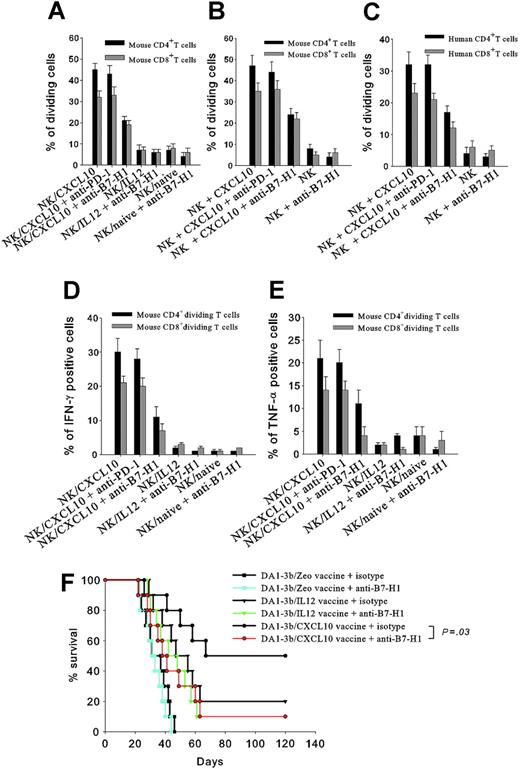
To explore the potential effect of B7-H1 overexpression, we cocultivated B7-H1+ NK cells isolated from mice vaccinated with DA1-3b/CXCL10 with naive CD4+ or CD8+ T cells. These B7-H1+ NK cells induced proliferation of CD4+ and CD8+ T cells, and this effect was inhibited by anti-B7-H1 antibody (Figure 7A). B7-H1+ NK cells obtained in vitro by incubation with recombinant CXCL10 produced similar results (Figure 7B). The same experiments performed with human NK cells induced proliferation of human CD4+ and CD8+ T cells (Figure 7C). Mouse B7-H1+ NK cells induced IFN-γ and TNF-α production in CD4+ and CD8+ T cells (Figure 7D-E), and similar results were obtained with human cells. In both humans and mice, B7-H1+ NK cells increased IL-10 production slightly, but did not affect IL-4 production (data not shown). Blocking PD-1, currently the only known receptor of B7-H1, did not affect the results, thus indicating that B7-H1 molecules expressed by NK cells stimulate T cells via another unknown receptor (Figure 7A-E).
Proliferation and cytokine secretion of T cells incubated with NK cells. (A) NK cells isolated from mice vaccinated with DA1-3b/CXCL10 cells (NK/CXCL10) or DA1-3b/IL12 cells (NK/CXCL10) or not vaccinated (NK/naive) were cocultured for 2 weeks with CFSE-labeled mouse CD4+ (▪) or CD8+ T cells (▦) and analyzed by flow cytometry for percentage of dividing cells. (B) Same procedure as in panel A but the NK cells were from naïve mice and incubated in vitro with 100 ng/mL recombinant mouse CXCL10 for 24 hours before coculture. (C) Human NK cells were incubated with 100 ng/mL recombinant human CXCL10 for 24 hours, then cocultured with CFSE-labeled human CD4+ (▪) or CD8+ T cells (▦) for 2 weeks and analyzed by flow cytometry for percentage of dividing cells. (D-E) Same procedure as panel A with mouse CD4+ (▪) or CD8+ T cells (▦) colabeled with CSFE and (D) an anti–IFN-γ or (E) anti–TNF-α antibody. (F) Effect of in vivo blocking of B7-H1 with anti-B7-H1 (colored lines and symbols) or control isotype (black lines and symbols) on overall survival of mice vaccinated with 106 irradiated leukemic cells (•, DA1-3b/CXCL10; ▴, DA1-3b/IL-12; or ▪, DA1-3b/Zeo) and then challenged with 105 wild-type DA1-3b leukemic cells. All experiments were performed in quadruplicate and repeated at least 3 times. Data represent mean and SD.
Proliferation and cytokine secretion of T cells incubated with NK cells. (A) NK cells isolated from mice vaccinated with DA1-3b/CXCL10 cells (NK/CXCL10) or DA1-3b/IL12 cells (NK/CXCL10) or not vaccinated (NK/naive) were cocultured for 2 weeks with CFSE-labeled mouse CD4+ (▪) or CD8+ T cells (▦) and analyzed by flow cytometry for percentage of dividing cells. (B) Same procedure as in panel A but the NK cells were from naïve mice and incubated in vitro with 100 ng/mL recombinant mouse CXCL10 for 24 hours before coculture. (C) Human NK cells were incubated with 100 ng/mL recombinant human CXCL10 for 24 hours, then cocultured with CFSE-labeled human CD4+ (▪) or CD8+ T cells (▦) for 2 weeks and analyzed by flow cytometry for percentage of dividing cells. (D-E) Same procedure as panel A with mouse CD4+ (▪) or CD8+ T cells (▦) colabeled with CSFE and (D) an anti–IFN-γ or (E) anti–TNF-α antibody. (F) Effect of in vivo blocking of B7-H1 with anti-B7-H1 (colored lines and symbols) or control isotype (black lines and symbols) on overall survival of mice vaccinated with 106 irradiated leukemic cells (•, DA1-3b/CXCL10; ▴, DA1-3b/IL-12; or ▪, DA1-3b/Zeo) and then challenged with 105 wild-type DA1-3b leukemic cells. All experiments were performed in quadruplicate and repeated at least 3 times. Data represent mean and SD.
Blocking B7-H1 in vivo
To evaluate the role of B7-H1 in CXCL10-induced antileukemic immunity, we injected anti-B7-H1–blocking antibodies, or a control isotype, into mice vaccinated with irradiated DA1-3b/CXCL10, DA1-3b/IL12, or control DA1-3b/Zeo cells, and then challenged immunity by injection of 105 live wild-type DA1-3b cells. Blocking B7-H1 partially abolished the prophylactic effect of DA1-3b/CXCL10 vaccine (P = .03, log-rank test), but had no significant effect on DA1-3b/Zeo and DA1-3b/IL12 groups (Figure 7F).
Discussion
In the DA1-3b mouse model of AML, injection of irradiated leukemic cells transduced by the chemokine CXCL10 led to a prophylactic immunity, with long-term memory, and cured pre-established leukemia in mice. This immunity was far more efficient than immunity induced by vaccines transduced with genes for IL-12, CD154, GM-CSF, or other vaccines we tested previously.2-4 The CXC10 vaccine cured 80% of animals, whereas the IL-12 vaccine cured only 40%. The effect of the CXCL10 vaccine was dose-dependent because injection of 106 cells led to better results than 105 cells, but injection of 107 cells was deleterious, possibly because a too large amount of chemokine might disrupt the trafficking of immune cells. Other chemokine-transduced vaccines also induce efficient antileukemic immunity. In the A20 mouse lymphoma/leukemia model, CCL3 cells transduced with GM-CSF or IL-2 were effective, but CCL3 cells alone were not.25 In C57BL/6 mice, CXCL12 transduction of C1498 leukemia also induced rejection of leukemic cells and cured pre-established leukemia.26 Thus, chemokines appear as interesting candidate genes for tumor vaccine strategies.
Eradication of dormant tumor cells is another aim of immunotherapy. Long-term persistence of tumor cells in patients in complete remission is common in human cancer and explains the occurrence of relapses years or decades later. Concerning the DA1-3b mouse model of AML, we recently reported that despite an effective antileukemic response induced by CD154- or IL-12–transduced leukemic cells, several hundred leukemic cells were detected in the spleen and the liver of mice 1 year after challenge.4 Those cells were still able to kill naive hosts, and they resisted CD8+ CTL-mediated lysis. In the present study of mice immunized with DA1-3b/CXCL10 cells, we sought persistent leukemic cells in every organ of mice that survived 1 year after challenge. We never detected any leukemic cells, which indicates the antileukemic immunity both improves survival rates and eradicates dormant tumor cells.
In vivo depletion experiments showed that this antileukemic immunity was partially dependent on CD4+ and CD8+ cells, but completely dependent on NK cells. In addition, blocking IFN-γ completely inhibited the vaccine effect but blocking TNF-α did not. The immune infiltrate at the vaccine site contained no B cells, and dendritic cells were detected only 2 weeks after vaccination, with a slightly higher proportion in mice vaccinated with DA1-3b/CXCL10 cells. DA1-3b/CXCL10 cells and DA1-3b/IL12 cells attracted T cells equally, but the former were more effective in attracting NK cells. An increased percentage of NK cells was also found in the spleen of mice vaccinated with DA1-3b/CXCL10 cells, even though the percentage of T cells was higher in the DA1-3b/IL12 group. Thus, it appears that DA1-3b/CXCL10 cells induce a massive recruitment of NK cells and modifications of lymphoid cell repartition in the spleen. The chemoattractant effect of CXCL10 on NK cells has been demonstrated in vitro, and CXCL10 expressed by thymic epithelial cells attracts NK-type cells.11,13 In our experiments, the NK cells that infiltrated the matrigel at the site of vaccination expressed the activating NK receptor LY49D, but not the inhibitory receptor LY49A. Using a rat NK cell line transfected with LY49D or LY49A, Inngjerdingen et al have shown that ligation of LY49D increased migration toward CXCL10, whereas ligation of LY49A decreased migration.12 Thus, CXCL10-transduced leukemia cell vaccines preferentially attract to the vaccine site NK cells that respond to this chemokine.
CD8+ CTLs isolated from mice vaccinated with DA1-3b/CXCL10 cells showed specific but lower lytic activity against leukemic cells than CTLs from the DA1-3b/IL12-vaccinated mice. In contrast, NK cells isolated from mice vaccinated with DA1-3b/CXCL10 showed higher lytic activity. In our previous experiments, we isolated persistent leukemic cells from the spleen of mice that survived the challenge.4 These cells showed resistance to CTL activity, proportional to the time they had spent in the host. However, NK cells isolated from DA1-3b/CXCL10-vaccinated mice killed these leukemic cells with equal efficacy regardless of whether the leukemic cells were isolated after 1, 3, or 12 months in the host. Thus, dormant tumor cells can escape to specific CTL activity but are killed by NK cells activated by CXCL10. We previously showed that dormant tumor cells inhibited CD8+ CTL-mediated lysis by overexpressing B7-H1 and B7.1.4 B7-H1 (also known as PD-L1) is a B7 family member and ligand for PD-1, a member of the CD28 family.27 B7-H1 can interact with receptors on CTLs and promote CTL death, and thus increased expression of B7-H1 might protect persistent leukemic cells from elimination. In the present experiments, addition of anti-B7-H1– and anti-B7.1–blocking antibodies did not assist NK cells to kill dormant tumor cells overexpressing B7-H1 and B7.1. NK cells express PD-1, the only known receptor of B7-H1, but no differences of expression could be found between the different vaccine groups. Several reports have shown that anti-PD-1–blocking antibodies do not reverse the inhibitory effect of B7-H1, suggesting that there is a second receptor for B7-H1, which might mediate its inhibitory effects on T cells.18,28 This hypothetical receptor is presumably either not expressed on NK cells or is inactive.
NK cells appear as key effectors of CXCL10-induced antileukemic immunity. However CD4+ and CD8+ T cells also play a role because depletion of these cells partially inhibited the antileukemic effect. In addition, the efficient memory immunity observed suggests that both innate and adaptive immunity control the leukemic disease. To determine if CXCL10 induced changes in NK cells that then allowed them to stimulate T cells, we analyzed costimulatory molecules in NK cells isolated from matrigel infiltrates, or from spleen, or incubated in vitro with recombinant CXCL10. Surprisingly, we found a strong expression of B7-H1 in NK cells exposed in vitro or in vivo to CXCL10. This B7-H1 expression was also obtained with the structurally related chemokines CXCL11 and CXCL9, which also bind to the CXCR3 receptor. CXCL12, which binds to the CXCR4 receptor, had the same effect. The effect was not restricted to CXC chemokines because IFN-γ and TNF-α also induced B7-H1. However, the tested CC chemokines did not modify the expression of B7-H1. IL-2 had no effect, indicating that nonspecific activation of NK cells does not induce B7-H1 expression. Blocking B7-H1 in vivo partially inhibited the vaccine effect of irradiated DA1-3b/CXCL10 but not of DA1-3b/IL12 or DA1-3b/Zeo controls. Thus, in our model, B7-H1 participates in the antileukemic immunity induced by CXCL10.
The role of B7-H1 in tumor escape from CTL activity has been described in several models, including ours. Recently, the generation of B7-H1–deficient mice has shown that B7-H1 expressed on antigen-presenting cells (APCs), on T cells, or on host tissue regulates T cells negatively.29 However, in nontumoral pathogenic models, B7-H1 apparently activates T cells. In a mouse model of diabetes, B7-H1 promotes autoimmune T-cell responses.30 In a model of colitis, blocking B7-H1 suppressed chronic intestinal inflammation.17 Thus, B7-H1 appears to have a dual activity. The first report on B7-H1 showed that it costimulated T-cell proliferation and induced IL-10 and IFN-γ production, whereas IL-4 remained unaffected.31 In our experiments, either mouse or human B7-H1+ NK cells stimulated the proliferation of CD4+ and CD8+ T cells and secretion of IFN-γ and TNF-α, whereas IL-10 was slightly increased and IL-4 was not modified. These effects appeared specific because blocking B7-H1 in vitro inhibited these stimulatory effects. Blocking PD-1, which is currently the only known receptor of B7-H1, had no effect on the results, thus indicating that B7-H1+ NK cells stimulate T cells via a second receptor, which might also mediate activating effects on T cells. Thus, it can be hypothesized that CXCL10 might also enhance T-cell response via B7-H1+ NK cells, in addition to enhancing NK cell cytotoxicity against leukemic cells and dormant tumor cells.
Matrigel infiltrates induced by CXCL10 were composed of both T cells and B7-H1+ NK cells. The proximity of these cells at the vaccine site might facilitate NK/T-cell interaction. The reason B7-H1–blocking antibodies abolish the vaccine effect of CXCL10 might be that other cells, besides NK cells, also express B7-H1. In our model, B7-H1 is also expressed on leukemic cells, but only after months of persistence.4 Thus, the injection of anti-B7-H1 antibodies into mice immediately after the last injection of leukemia vaccine is unlikely to interfere with long-term persistence of leukemic cells. B7-H1 also occurs naturally in a range of tissues, T cells, and APCs, and their function could be affected by anti-B7-H1.32,33 However, the demonstration of inhibitory function of B7-H1 on T cells and APCs, using B7-H1–deficient mice, argues against a positive role for the B7-H1 molecules expressed on these cells in antitumor immunity.29 Our observation that B7-H1 is expressed on NK cells after exposure to inflammatory chemokines might indicate that B7-H1+ NK cells might constitute a new mediator of T-cell activation. We observed in human NK cells the same induction of B7-H1 expression by in vitro exposure to CXC chemokines. More specifically, the 2 major subsets of NK cells in humans were differentially sensitive to CXCL10. The CD56brightCD16dim NK cells showed a stronger induction of B7-H1 expression than CD56dimCD16bright cells. The majority of human NK cells are phenotypically CD56dimCD16bright and are more highly cytotoxic than the other subset, which represent less than 10% of NK cells.34 CD56brightCD16dim NK cells express high levels of CXCR3, the receptor of CXCL1035 , and perhaps this is why they produce higher levels of B7-H1. CD56brightCD16dim NK cells express homing receptors and have been identified in lymph nodes and may therefore regulate T cells and dendritic cells.36 Our finding that these NK cells may also express B7-H1 is another argument for a regulatory role.
In summary, we identified CXCL10 as a powerful chemokine able to induce a strong antileukemic response against both leukemic disease and long-term persistent leukemic cells via enhanced NK cell cytotoxicity and B7-H1–mediated immunity. NK cells are key effectors of the graft-versus-leukemia effect after allogeneic stem cell transplantation. Our results indicate that CXCL10-activated NK cells might also mediate an efficient autologous immune response in human AML.
Prepublished online as Blood First Edition Paper, November 9, 2004 DOI 10.1182/blood-2004-09-3458.
Supported by the Ligue Contre le Cancer (Comité du Septentrion), the Association de Recherche sur le Cancer, the Groupements des Entreprises Françaises dans la Lutte contre le Cancer (GEFLUC), the Fondation contre la Leucémie, the Institut de Recherche sur le Cancer de Lille, and the Centre Hospitalier Universitaire of Lille, France. A.S. is the recipient of a grant from the Region Nord-Pas-de-Calais/Centre Hospitalier Régionale Universitaire (CHRU) de Lille.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal