Abstract
Endothelial cells normally form a dynamically regulated barrier at the blood-tissue interface, and breakdown of this barrier is a key pathogenic factor in inflammatory disorders such as sepsis. Pro-inflammatory signaling by the blood coagulation protease thrombin through protease activated receptor-1 (PAR1) can disrupt endothelial barrier integrity, whereas the bioactive lipid sphingosine 1-phosphate (S1P) recently has been demonstrated to have potent barrier protective effects. Activated protein C (APC) inhibits thrombin generation and has potent anti-inflammatory effects. Here, we show that APC enhanced endothelial barrier integrity in a dual-chamber system dependent on binding to endothelial protein C receptor, activation of PAR1, and activity of cellular sphingosine kinase. Small interfering RNA that targets sphingosine kinase-1 or S1P receptor-1 blocked this protective signaling by APC. Incubation of cells with PAR1 agonist peptide or low concentrations of thrombin (∼ 40 pM) had a similar barrier-enhancing effect. These results demonstrate that PAR1 activation on endothelial cells can have opposite biologic effects, reveal a role for cross-communication between the prototypical barrier-protective S1P and barrier-disruptive PAR1 pathway, and suggest that S1P receptor-1 mediates protective effects of APC in systemic inflammation.
Introduction
A monolayer of endothelial cells separates the blood from underlying tissues, and regulation of this barrier's permeability controls the exchange of proteins and cells at the blood-tissue interface. Breakdown of this barrier plays a key role in inflammatory disorders such as sepsis. The blood coagulation protease thrombin has pro-inflammatory effects on endothelial cells and is a well-characterized barrier disruptive factor.1 In contrast, the bioactive lipid sphingosine 1-phosphate (S1P) recently has been demonstrated to have potent barrier protective effects.2,3 The opposite effects of thrombin and S1P on endothelial barrier integrity are both mediated by G-protein–coupled receptors, that is, protease-activated receptors (PARs)4 and S1P receptors,5 respectively. Current information indicates that PAR1 is the main receptor responsible for thrombin signaling in endothelial cells, whereas endothelial cell PAR2 is not activated by thrombin but by several other proteases including trypsin and mast cell tryptase.6,7 Thrombin activity is increased in inflammation, and its generation is controlled by the anticoagulant protein C pathway in a negative feedback loop.8,9 Activated protein C (APC) has potent anti-inflammatory effects in animal models that are incompletely understood and at least in part, independent of its anticoagulant function,10-13 and recombinant human APC was approved to treat patients with severe sepsis.14 We have demonstrated previously that dependent upon binding of APC to endothelial cell protein C receptor (EPCR), the prototypical thrombin receptor PAR1 mediates gene induction by APC in endothelial cells,15 and subsequent studies have implicated this signaling pathway in neuroprotective effects of APC in vivo.13,16 However, it remains a major question how the same receptor PAR1 that mediates pro-inflammatory thrombin signaling can mediate protective effects of APC.
Materials and methods
Agonists, inhibitors, and antibodies
Thrombin and specific agonist peptides for PAR1 (TFLLRNPNDK) and PAR2 (SLIGRL) were as described.15,17 Human APC was from Haematologic Technologies (Essex Junction, VT). All experiments involving stimulation with APC included hirudin (Calbiochem, La Jolla, CA) to block any thrombin signaling. Control experiments demonstrated that hirudin alone had no effect in any of our assays. S1P and the competitive sphingosine kinase inhibitors DL-threo-dihydrosphingosine (DHS) and N, N-dimethylsphingosine (DMS) were from Biomol (Plymouth Meeting, PA). The S1P1 selective agonist SEW287118 was kindly provided by Dr Hugh Rosen. Ki values for SK inhibition are approximately 5 to 18 μM for DHS and 2 to 5 μM for DMS.19 Recombinant vascular endothelial growth factor165 (VEGF165) was from Calbiochem. PAR1 cleavage-blocking monoclonal antibodies ATAP2 and WEDE15 have been characterized previously and were used in combination at 10 μg/mL and 25 μg/mL, respectively.15,17,20 Monoclonal rat anti–EPCR RCR-92 (nonblocking) and RCR-252 (blocking) were provided by Dr Kenji Fukudome and were used at 25 μg/mL.21
Cell culture
Primary human umbilical vein endothelial cells (HUVECs) (Clonetics, Walkersville, MD) were maintained at 37°C in a 5% CO2 incubator in EGM-2 media (basal endothelial cell media supplemented with hydrocortisone, bovine brain extract, human epidermal growth factor, gentamicin/amphotericin, and 10% fetal bovine serum). They were passaged for no more than 7 generations. The transformed human endothelial cell line EA.hy92622 was provided by Dr C. Edgell (University of North Carolina at Chapel Hill, NC). Cells were grown to confluence in a humidified atmosphere at 37°C in Dulbecco modified eagle medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Omega Scientific, Tarzana, CA). EA.hy926 cells express EPCR23 and have been used previously to study effects of APC on endothelial cell apoptosis.24,25
Permeability assay
Macromolecular monolayer permeability was analyzed in a dual-chamber system using Evans blue-labeled bovine serum albumin (BSA). Endothelial cells were plated at an average density of 5 × 104 cells/well on gelatin-coated (HUVEC) or untreated (EA.hy926 cells) transwell polycarbonate membranes of 3-μm pore size and 12-mm diameter (Costar, Corning, NY). The upper and lower chambers were filled with 500 μL and 1500 μL growth medium, respectively. Cells were grown until subconfluence (3 days) or until confluence was obtained (4-6 days). The day before performing the experiments, medium was replaced with fresh growth medium. Permeability was assayed using 0.67 mg/mL Evans blue (Sigma, St Louis, MO) diluted in growth medium containing 4% BSA (Calbiochem) as described previously.26 Briefly, fresh growth medium was added to the lower chamber, and the medium in the upper chamber was replaced with Evans blue/BSA. After 10 minutes the optical density at 650 nm was measured in a 1: 3 diluted 50 μL sample from the lower chamber. For agonist treatment of the monolayers, medium was removed and agonists were added for 10 minutes in serum-free medium followed by analysis of permeability. In experiments using S1P or SEW2871, medium was changed to basal medium containing 4% charcoal dextran-treated fetal bovine serum (Omega Scientific) 30 minutes before the experiment, and the agonists were added in basal medium containing 0.4% fatty acid-free BSA (Calbiochem).
Analysis of kinase activation
Mitogen-activated protein (MAP) kinase phosphorylation was analyzed by Western blotting using an anti–phospho Erk1/2 antibody (#9101; Cell Signaling Technology, Beverly, MA) as described.15 Staining with antiactin (Sigma) served as loading control.
RNA interference
Chemically synthesized, double-stranded small interfering RNA (siRNA) with 19-nucleotide duplex RNA and 2-nucleotide 3′ dTdT overhangs were obtained from Ambion (Austin, TX) (targeting S1P1 and S1P3) or Qiagen (Valencia, CA) (targeting SK1). The targeted sequences were 5′-GGAGAACAGCATTAAACTG-3′ (S1P1), 5′-GGTCAACATTCTGATGTCT-3′ (S1P3), and 5′-GGGCAAGGCCTTGCAGCTC-3′ (SK1). EA.hy926 cells were plated at 5 × 104 cells/well into transwell chambers and transfected 24 hours later in fresh growth medium with 2 μg siRNA duplexes per well using RNAiFect (Qiagen) according to modified manufacturer's instructions. Briefly, 12 μL per well of the RNAiFect transfection reagent was added to 88 μL per well of growth medium containing 2 μg siRNA. After vortexing and a 15-minute incubation at room temperature, the mixture (100 μL/well) was added dropwise to the upper transwell chamber. The cells were used for experiments 2 to 3 days after transfection. Transfection medium was replaced with fresh growth medium on the day before the experiment.
Statistical analysis
A 2-sample 2-tailed homoscedastic t test was used to calculate the indicated P values.
Results
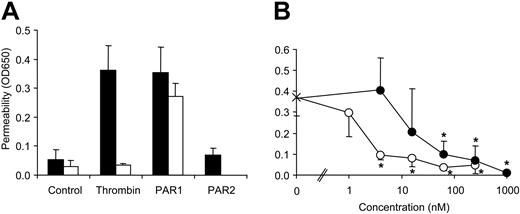
To analyze effects of APC on endothelial cell permeability, we established a dual chamber system measuring albumin flux. Consistent with previous results,27,28 treatment of a confluent monolayer of HUVECs with thrombin or PAR1 but not with PAR2 agonist peptide led to a rapid increase of permeability in our system (Figure 1A). Cleavage-blocking anti-PAR1 inhibited responses to thrombin but not to the cleavage-independent PAR1 agonist peptide, demonstrating that thrombin-induced hyperpermeability requires PAR1 activation. The prototypical barrier enhancing S1P signaling pathway was functional in our system because preincubation of confluent cells with S1P blocked PAR1-mediated hyperpermeability (Figure 1B). To test whether S1P1 activation alone is sufficient for barrier protective signaling, we used the S1P1 selective agonist SEW2871. Again PAR1-mediated hyperpermeability was blocked by preincubation with SEW2871 (Figure 1B), and half-maximal inhibition was observed at concentrations close to the 13 nM published EC50 for S1P1 activation.18 Thus, S1P1 agonism alone is sufficient for barrier protective S1P signaling in endothelial cells. Similar results were obtained using the HUVEC-derived immortalized cell line EA.hy926, and we used this cell line to analyze effects of APC on endothelial cell barrier function.
Effect of agonists for PARs and S1P receptors on endothelial barrier function. (A) Confluent HUVECs were incubated for 10 minutes in a dual-chamber system with the indicated agonists (5 nM thrombin, 10 μM PAR1 agonist TFLLRN-PNDK, 100 μM PAR2 agonist SLIGRL) in the absence (▪) or presence (□) of cleavage-blocking anti-PAR1 (combination of ATAP2 and WEDE15), and permeability was determined using Evans blue–labeled albumin in the upper chamber and measuring the optical density at 650 nm (OD650) in the lower chamber (mean ± SD, n = 3). (B) Confluent HUVECs were incubated for 10 minutes with the indicated concentrations of S1P (○) or SEW2871 (•) followed by a 10-minute incubation with 10 μM PAR1 agonist (means ± SD, n = 4, *P < .005 compared to no agonist).
Effect of agonists for PARs and S1P receptors on endothelial barrier function. (A) Confluent HUVECs were incubated for 10 minutes in a dual-chamber system with the indicated agonists (5 nM thrombin, 10 μM PAR1 agonist TFLLRN-PNDK, 100 μM PAR2 agonist SLIGRL) in the absence (▪) or presence (□) of cleavage-blocking anti-PAR1 (combination of ATAP2 and WEDE15), and permeability was determined using Evans blue–labeled albumin in the upper chamber and measuring the optical density at 650 nm (OD650) in the lower chamber (mean ± SD, n = 3). (B) Confluent HUVECs were incubated for 10 minutes with the indicated concentrations of S1P (○) or SEW2871 (•) followed by a 10-minute incubation with 10 μM PAR1 agonist (means ± SD, n = 4, *P < .005 compared to no agonist).
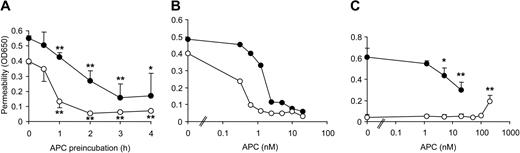
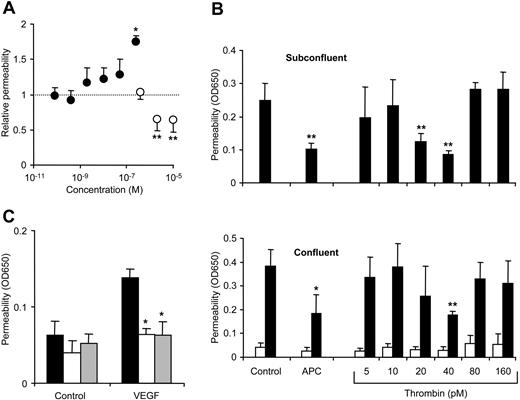
Thrombin-mediated hyperpermeability was reduced when the cells were pretreated with 20 nM APC with significant protection already after 1 hour and maximal effect after 3 hours' pretreatment (Figure 2A). Thrombin-induced barrier dysfunction was transient, and APC accelerated reconstitution of barrier function. APC had a similar protective effect on PAR1 agonist peptide-mediated hyperpermeability (not shown). Because median therapeutic steady-state plasma levels of recombinant APC in sepsis patients were found to be only 0.8 nM,29 we tested lower APC concentrations in our system (Figure 2B). Endothelial cell barrier function was enhanced by 0.5 to 2 nM APC, thus concentrations effective in our model are comparable to therapeutic levels in APC-treated patients. Repeat experiments demonstrated that when cells were less responsive to thrombin, APC was protective at lower concentrations, suggesting that cells with better established cell–cell contacts are more responsive to barrier enhancement by APC. Very high APC concentrations (200 nM) rapidly induced a slight increase in basal permeability consistent with previous results,30 but APC did not affect basal permeability at concentrations that significantly reduced thrombin-mediated hyperpermeability upon 3 hours of preincubation (Figure 2C).
APC pretreatment reduces thrombin-mediated hyperpermeability. (A) Confluent EA.hy926 cells were incubated for the indicated periods with 20 nM APC followed by a 10-minute incubation with thrombin. Permeability was tested after 10 minutes (•) and 20 minutes (○) (mean ± SD, n = 3, *P < .05 and **P < .005 compared to no APC). (B) Confluent cells were incubated for 3 hours with the indicated concentrations of APC followed by a 10-minute incubation with thrombin. (•) and (○) represent results from 2 of 4 independent experiments. (C) In parallel experiments confluent cells were either incubated for 3 hours with the indicated concentrations of APC followed by a 10-minute incubation with thrombin (•) or for 3 hours with medium control followed by a 10-minute incubation with the indicated concentrations of APC (○) (mean ± SD, n = 4, *P < .05 and **P < .005 compared to no APC).
APC pretreatment reduces thrombin-mediated hyperpermeability. (A) Confluent EA.hy926 cells were incubated for the indicated periods with 20 nM APC followed by a 10-minute incubation with thrombin. Permeability was tested after 10 minutes (•) and 20 minutes (○) (mean ± SD, n = 3, *P < .05 and **P < .005 compared to no APC). (B) Confluent cells were incubated for 3 hours with the indicated concentrations of APC followed by a 10-minute incubation with thrombin. (•) and (○) represent results from 2 of 4 independent experiments. (C) In parallel experiments confluent cells were either incubated for 3 hours with the indicated concentrations of APC followed by a 10-minute incubation with thrombin (•) or for 3 hours with medium control followed by a 10-minute incubation with the indicated concentrations of APC (○) (mean ± SD, n = 4, *P < .05 and **P < .005 compared to no APC).
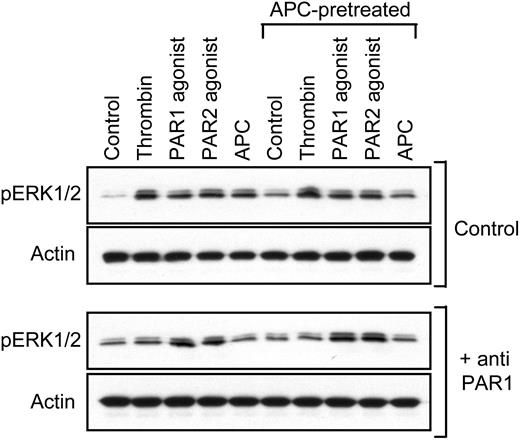
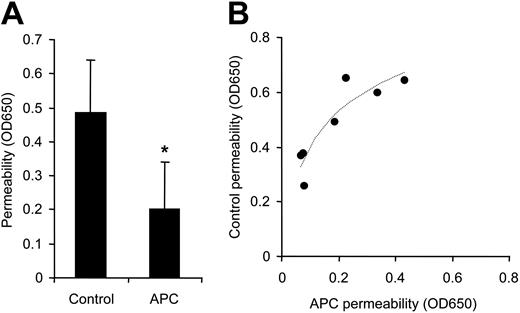
Because APC can cleave PAR1, we tested whether preincubation with APC can desensitize Erk1/2 phosphorylation by thrombin using similar conditions as for the permeability studies. Figure 3 demonstrates that phospho-Erk1/2 was induced by APC dependent upon PAR1 cleavage, but APC-pretreated cells still responded to PAR1-dependent thrombin signaling. These results, together with the prolonged time course for protective APC signaling, indicate that the blocking effect of APC on PAR1-mediated hyperpermeability is not caused by desensitization on the receptor level. Consistent with this conclusion, APC incubation reduced permeability in subconfluent monolayers of cells in the absence of hyperpermeability-inducing agonists to on average 41% of control levels (Figure 4A). Again, APC more efficiently sealed cell–cell contacts in monolayers with better barrier function (Figure 4B).
APC pretreatment does not lead to PAR1 desensitization. Cells were incubated for 3 hours with control or 5 nM APC (APC-pretreated) followed by a 10-minute stimulation with the indicated agonists in the absence and presence of cleavage-blocking anti-PAR1. Extracellular signal-related kinase (Erk)–1/2 phosphorylation was analyzed by Western blotting. A typical experiment is shown.
APC pretreatment does not lead to PAR1 desensitization. Cells were incubated for 3 hours with control or 5 nM APC (APC-pretreated) followed by a 10-minute stimulation with the indicated agonists in the absence and presence of cleavage-blocking anti-PAR1. Extracellular signal-related kinase (Erk)–1/2 phosphorylation was analyzed by Western blotting. A typical experiment is shown.
APC enhances barrier integrity in subconfluent cells. In 7 independent experiments subconfluent cells were incubated for 3 hours with control or 5 nM APC followed by permeability analysis. Means ± SD are shown in panel A (*P < .005), and results from the individual experiments with logarithmic regression line are plotted in panel B.
APC enhances barrier integrity in subconfluent cells. In 7 independent experiments subconfluent cells were incubated for 3 hours with control or 5 nM APC followed by permeability analysis. Means ± SD are shown in panel A (*P < .005), and results from the individual experiments with logarithmic regression line are plotted in panel B.
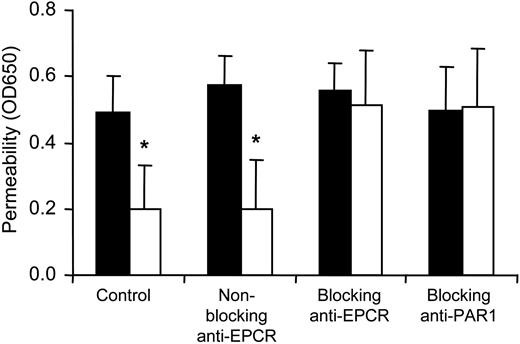
Antibodies that block either binding of APC to EPCR or cleavage of PAR1 abolished the barrier-protective effect of APC both in subconfluent (Figure 5) and in confluent (not shown) cells. Thus, the receptor system that mediates APC-dependent gene induction in endothelial cells also mediates barrier protection. Most importantly, activation of the same receptor PAR1 that mediates the rapid thrombin-induced hyperpermeability is also required for barrier-protective signaling by APC, raising the question whether PAR agonists other than APC can induce barrier-protective signaling. Incubation for 3 hours in the presence of a large range of thrombin concentrations (80 pM-250 nM) did not lead to enhanced barrier function in subconfluent cells (Figure 6A). Permeability was significantly higher only at the very highest thrombin concentration, in agreement with the well-established observation that thrombin-mediated hyperpermeability is transient.31 In contrast, 3 hours of incubation with 2 or 10 μM PAR1 agonist peptide enhanced barrier integrity (Figure 6A). Thrombin at nanomolar concentrations is likely a much faster acting PAR1 activator than APC and also the PAR1 agonist peptide, and we analyzed whether lower concentrations of thrombin can be barrier protective. Because thrombin is susceptible to inactivation by residual inhibitors from serum, we established a serum-free system for these assays. Figure 6B demonstrates that a 3-hour incubation with thrombin at concentrations of 20 to 40 pM indeed reduced permeability in subconfluent cells and diminished the hyperpermeability in response to thrombin in confluent cells. Thrombin at 40 pM was similarly efficient as APC in inducing barrier-enhancing signaling. Concentrations of thrombin at 10 pM or lower and 80 pM or higher were ineffective in both subconfluent and confluent systems. Preincubation with 80 or 160 pM thrombin did not desensitize the subsequent hyperpermeability response to 5 nM thrombin (Figure 6B), thus, protection by 40 pM thrombin in the confluent cells is not mediated by receptor desensitization. In agreement with this conclusion, preincubation with both APC and 40 pM thrombin blocked in our confluent cell system the modest barrier disruptive effect of VEGF (Figure 6C).
Role of EPCR and PAR1 in barrier-enhancing signaling by APC. Subconfluent cells were incubated for 3 hours in the absence (▪) or presence (□) of 5 nM APC. The indicated antibodies were added 10 minutes prior to APC (means ± SD, n = 3, *P < .05 compared to no APC).
Role of EPCR and PAR1 in barrier-enhancing signaling by APC. Subconfluent cells were incubated for 3 hours in the absence (▪) or presence (□) of 5 nM APC. The indicated antibodies were added 10 minutes prior to APC (means ± SD, n = 3, *P < .05 compared to no APC).
Effect of thrombin and PAR1 agonist peptide on barrier integrity. (A) Subconfluent cells were incubated for 3 hours with the indicated concentrations of thrombin (•) or PAR1 agonist peptide (○) (means ± SD, n = 3 for thrombin, n = 7 for PAR1, *P < .05 and **P < .005 compared to the lowest agonist concentration). (B) Subconfluent (top) or confluent (bottom) cells were incubated in serum-free growth medium containing 0.4% BSA for 3 hours with APC or the indicated concentrations of thrombin followed by permeability test in the subconfluent cells. In confluent cells permeability was tested before (□) and after an additional 10-minute incubation with 5 nM thrombin (▪) (means ± SD, n = 4, *P < .05 and **P < .005 compared to control). (C) Confluent cells were incubated in serum-free growth medium for 3 hours with carrier control (▪), APC (□), or 40 pM thrombin (▦) followed by a 30-minute incubation with control or 10 nM VEGF (means ± SD, n = 4, *P < .005 compared to carrier control).
Effect of thrombin and PAR1 agonist peptide on barrier integrity. (A) Subconfluent cells were incubated for 3 hours with the indicated concentrations of thrombin (•) or PAR1 agonist peptide (○) (means ± SD, n = 3 for thrombin, n = 7 for PAR1, *P < .05 and **P < .005 compared to the lowest agonist concentration). (B) Subconfluent (top) or confluent (bottom) cells were incubated in serum-free growth medium containing 0.4% BSA for 3 hours with APC or the indicated concentrations of thrombin followed by permeability test in the subconfluent cells. In confluent cells permeability was tested before (□) and after an additional 10-minute incubation with 5 nM thrombin (▪) (means ± SD, n = 4, *P < .05 and **P < .005 compared to control). (C) Confluent cells were incubated in serum-free growth medium for 3 hours with carrier control (▪), APC (□), or 40 pM thrombin (▦) followed by a 30-minute incubation with control or 10 nM VEGF (means ± SD, n = 4, *P < .005 compared to carrier control).
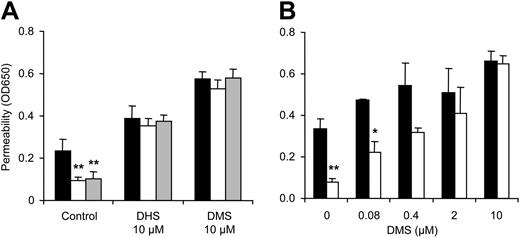
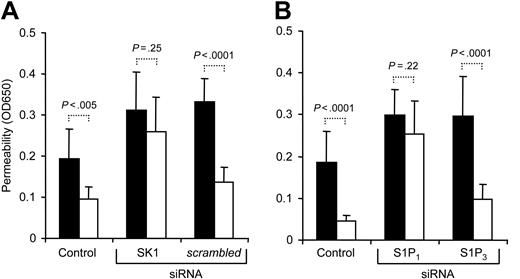
Based upon the established barrier protective efficacy of S1P signaling (Figure 1B), we hypothesized that PAR1-dependent APC signaling may lead to activation of cellular sphingosine kinase (SK) and generation of S1P, which in turn enhances barrier integrity. Co-incubation with 10 μM DL-threo-dihydrosphingosine (DHS) or N, N-dimethylsphingosine (DMS), potent competitive inhibitors of SK, indeed blocked barrier enhancement by APC and 40 pM thrombin (Figure 7A), and the blocking effect was dependent on the inhibitor concentration (Figure 7B). SK inhibitor treatment alone increased permeability of the monolayer consistent with the concept that blocking basal SK may reduce tonic activity in an autocrine or paracrine barrier-enhancing pathway. Control experiments demonstrated that SK inhibitor-treated cells still responded to barrier-enhancing effects of exogenous S1P (not shown). Transfection with siRNA targeting sphingosine kinase-1 (SK1) blocked the barrier-enhancing signaling of APC, whereas scrambled control siRNA was without effect (Figure 8A), implicating SK1 as an essential downstream mediator in this pathway. In view of our finding that S1P1 stimulation is sufficient for barrier protective signaling by exogenous S1P, these results suggest that autocrine or paracrine signaling of SK1-generated S1P through S1P1 may mediate barrier protection by APC. However, intracellularly generated S1P has been shown to affect cell growth and survival independent of cell surface receptors,32 and it remained possible that endogenously generated S1P may affect barrier function independent of S1P1. No specific inhibitors for S1P1 have been reported so far, and we used siRNA to down-regulate S1P1 expression. We hypothesized that even if S1P1 expression is efficiently down-regulated in only a fraction of the cells, APC's ability to enhance integrity of the monolayer should be significantly reduced. As shown in Figure 8B, targeting S1P1 but not S1P3 almost completely blocked barrier enhancement by APC. Taken together, these results indicate that both SK1 and S1P1 are essential components in a novel PAR1-dependent signaling cascade that leads to barrier protection by APC and low concentrations of thrombin (Figure 9).
Role of sphingosine kinase in barrier-enhancing signaling by APC and thrombin. Subconfluent cells were incubated in serum-free growth medium containing 0.4% BSA (A) or in full-growth medium (B) for 3 hours with carrier control (▪) or with APC (□) or 40 pM thrombin (▦). The indicated concentrations of sphingosine kinase inhibitors DHS or DMS were added 10 minutes prior to the agonists (means ± SD, n = 3, *P < .05 and **P < .005 compared to carrier control).
Role of sphingosine kinase in barrier-enhancing signaling by APC and thrombin. Subconfluent cells were incubated in serum-free growth medium containing 0.4% BSA (A) or in full-growth medium (B) for 3 hours with carrier control (▪) or with APC (□) or 40 pM thrombin (▦). The indicated concentrations of sphingosine kinase inhibitors DHS or DMS were added 10 minutes prior to the agonists (means ± SD, n = 3, *P < .05 and **P < .005 compared to carrier control).
Role of S1P1 in barrier-enhancing signaling by APC. Subconfluent untransfected cells (control) or cells transfected with siRNA targeting the indicated genes were incubated for 3 hours with carrier control (▪) or 5nMAPC(□) followed by analysis of permeability (means ± SD, n = 8 with 4 independent transfections, P values are indicated).
Role of S1P1 in barrier-enhancing signaling by APC. Subconfluent untransfected cells (control) or cells transfected with siRNA targeting the indicated genes were incubated for 3 hours with carrier control (▪) or 5nMAPC(□) followed by analysis of permeability (means ± SD, n = 8 with 4 independent transfections, P values are indicated).
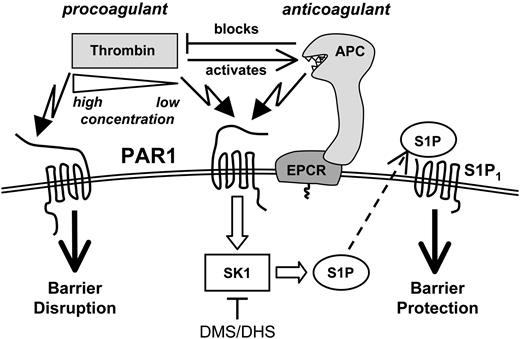
Dual role of PAR1. PAR1-dependent signaling by the interdependent procoagulant and anticoagulant proteases thrombin and APC can have opposite effects on endothelial barrier integrity. Barrier protection by APC or low concentrations of thrombin is mediated by sphingosine kinase-1 activity and crossactivation of S1P1 signaling.
Dual role of PAR1. PAR1-dependent signaling by the interdependent procoagulant and anticoagulant proteases thrombin and APC can have opposite effects on endothelial barrier integrity. Barrier protection by APC or low concentrations of thrombin is mediated by sphingosine kinase-1 activity and crossactivation of S1P1 signaling.
Discussion
These results demonstrate that barrier protection by APC proceeds via unanticipated crosstalk between the prototypical barrier-disruptive PAR1 and barrier-protective S1P signaling pathway. PAR1 activation by higher concentrations of thrombin leads to barrier disruption, whereas PAR1 activation by APC leads to S1P pathway-dependent barrier protective signaling. Thus, activation of the same receptor PAR1 on the same cell type can have opposite biologic effects, and cross-talk between receptor systems contributes to a balanced regulation, in which activation of systems can have both deleterious and protective effects. Our results implicate specifically SK1 and S1P1 in the barrier-protective downstream signaling cascade from PAR1. Mammalian cells express 2 isoforms of SK, SK1 and SK2, and the finding that down-regulation of SK1 alone blocks barrier enhancement is consistent with previous data that SK1 but not SK2 plays an important role in cellular signaling pathways.32-34 Endothelial cells express S1P receptors S1P1 and S1P3.3 In view of recent data that down-regulation of S1P1 by expression of an antisense oligonucleotide significantly diminishes S1P-mediated barrier enhancement,35 our data using an S1P1-specific agonist indicate that S1P1 agonism is not only necessary, but also sufficient for barrier protective S1P signaling in endothelial cells.
The finding that the PAR1 agonist peptide and thrombin at low concentrations of approximately 40 pM had barrier-protective effects comparable to APC suggests that barrier disruption and activation of S1P pathway signaling may be directly linked to the rate of PAR1 activation. Thrombin is barrier disruptive at higher concentrations but protective below its EC50 of 50 pM for PAR1 activation.36 APC is a poor agonist for PAR1 and leads to barrier protection at physiological concentrations, whereas only unphysiologically high APC concentrations can induce barrier disruption. In addition, these results implicate that although APC-PAR1 signaling is EPCR dependent, EPCR co-signaling is not required for the S1P pathway crossactivation that leads to barrier protection. Thus, thrombin can affect endothelial barrier properties in at least 3 different ways, adding additional complexity to “the thrombin paradox”37 : (1) higher thrombin concentrations disrupt barrier integrity through PAR1 activation, whereas a low thrombin concentration can induce barrier-protective signaling through the same receptor either (2) directly or (3) indirectly through APC generation. Due to this complexity it is likely that the relative importance of different pathways in vivo will depend on the extent and localization of thrombin generation as well as the availability of components of the protein C system. Thrombin-PAR1 signaling has been shown to be proinflammatory in a mouse model of glomerulonephritis.38 However, patients with the heterozygous factor V Leiden mutation who are expected to generate more thrombin in vivo have a survival advantage in severe sepsis.39 It is currently unclear whether this protection is mediated by direct thrombin signaling or indirectly by activation of the protein C pathway. Data from a mouse model of endotoxemia may suggest the latter,39 however, it will be challenging to determine the role of different pathways in animal models because thrombin and APC levels are interdependent in vivo, and the same receptor PAR1 may have opposite roles.
A recent report described effects of APC on endothelial cell permeability without addressing the mechanism of these effects.30 It is possible that crossactivation of S1P signaling is the underlying mechanism for other cellular effects of APC. Given the established antiapoptotic functions of SK1 and S1P,34,40 it is an attractive hypothesis that S1P signaling mediates the PAR1-dependent antiapoptotic effects of APC described in several studies.13,16,24,41 In one of these studies the PAR1 agonist peptide had significant antiapoptotic activity,13 whereas it was without effect in another study.24 In an interesting parallel of our results for barrier properties, thrombin-PAR1 signaling has been demonstrated to have bimodal concentration-dependent effects on growth and apoptosis in tumor cell lines42 and neuronal cells.43 Furthermore, the gene for the important immunomodulator monocyte chemoattractant protein-1 (MCP-1) is induced by PAR1 and APC,15 and in view of the recently established role of the S1P pathway in MCP-1 induction by tumor necrosis factor-α,44 it is possible that MCP-1 induction by PAR1 agonists is also mediated by the S1P pathway.
It is presently unknown whether effects on vascular permeability may play a role in APC's beneficial effects in patients with severe sepsis. Recombinant APC treatment was associated with lower usage of vasopressors,45 and although many different pathophysiological factors can lead to reduced blood pressure in septic patients, it seems reasonable to speculate that enhancement of the endothelial cell barrier by APC may contribute to the reduced vasopressor requirement. Our identification of S1P1 as an essential link in this protective signaling cascade suggests that direct S1P1 agonism may benefit patients with severe sepsis and possibly other inflammation-mediated pathologies. Very recently, non–subtype-specific S1P receptor agonists have been shown to have protective effects in a model of endotoxin-induced inflammatory lung injury,46 and specific S1P1 agonism may be equally effective while avoiding some side effects of nonsubtype specific agonists.18 It is possible that sphingosine kinase activities in endothelial cells represent an independent risk factor in sepsis, and potentially determination of these activities could predict which patients are likely to represent the subset who benefit from recombinant APC therapy.
Induction of barrier-protective S1P signaling by the barrier-disruptive PAR1 may have originated as negative feedback that enhances resealing of cell–cell contacts. Thrombin and APC are closely related serine proteases that developed different activation mechanisms and substrate specificities and became linked in a negative feedback loop after a gene duplication event in the early evolution of vertebrate blood coagulation. However, both proteases retained their ability to activate PAR1. The thrombin-PAR1 interaction through direct binding of PAR1's hirudin-like domain to thrombin's exosite 14 was optimized for the rapid and efficient receptor activation that is required for hemostasis after injury and that leads to barrier-disruptive signaling. On the other hand, APC may have only the ability to induce the barrier-protective S1P-dependent signaling at physiological concentrations because activation of PAR1 by APC is less efficient and restricted by its dependence on EPCR. Thus, in addition to its well-established role in preventing the spreading of thrombosis by down-regulating thrombin formation in a negative feedback loop, APC may have a similar role in preventing the spreading of barrier dysfunction and inflammation by signaling through the same receptor that is used by thrombin for proinflammatory signaling.
In conclusion, our results demonstrate that activation of the same G-protein–coupled receptor on the same cell type can have opposite biologic effects: they provide a novel SK1-dependent link between PAR1 and S1P1, and they suggest that S1P1 agonism may be an attractive concept in the treatment of systemic inflammation.
Prepublished online as Blood First Edition Paper, December 30, 2004; DOI 10.1182/blood-2004-10-3985.
Supported by National Institutes of Health grants HL 73318 (M.R.), a Junior Faculty Scholar Award from the American Society of Hematology (M.R.), and an Austrian fellowship, Erwin Schrödinger-Auslandsstipendium J2413-B13 (C.F.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs L. Brass, C. Edgell, K. Fukudome, and H. Rosen for invaluable reagents and Dr H. Rosen for many very helpful discussions. We also thank Jeffrey Sorokin for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal