A recent paper by Hakobyan et al1 in Blood has demonstrated that iron stimulates synovial cell division through oncogene (mdm2) activation in models of hemophilic arthropathy. This paper was the topic of an Inside Blood comment by Abshire,2 who stated that it would be important to identify other genetic factors explaining the heterogeneity of hemophilic arthropathy. In this letter we present relevant results of the severity of arthropathy associated with mutations in the HFE gene, namely C282Y and H63D, in 34 hemophilia patients. The results indicate that severity of the arthropathy measured both by number of hemarthrosis per year and number of affected joints is associated with the presence of the mutations, particularly with C282Y. Mechanisms may involve abnormal circulating iron levels in C282Y carriers and/or an increased efflux of iron from macrophages in affected joints.
Wen et al3 and Hakobyan et al1 have recently identified changes in oncogene expression in models of hemophilic arthropathy as the possible molecular basis of the effect of iron on synovial cell division originally described by Nishiya4 in rheumatoid arthritis patients. In an Inside Blood, the need to look for additional genetic factors contributing to the establishment of hemophilic arthropathy was discussed.2
We recently concluded a pilot study of the frequency of HFE mutations in a group of 34 hemophilia patients aged between 20 and 71 years, diagnosed before 1985 and followed up in Santo António General Hospital in Porto. The study design was approved by the Hospital Ethical Committee. Informed consent was provided according to the Declaration of Helsinki. All patients were genotyped for C282Y and H63D mutation as described.5 Allele frequencies of the C282Y (6 heterozygous and 2 compound heterozygous for C282Y and H63D) and H63D (12 heterozygous and 1 homozygous) mutations in this group of patients were 0.118 and 0.235, respectively, as opposed to the reported allele frequencies in a local control population (0.032 for the C282Y, P = .0009; and 0.198 for the H63D, P not significant).5
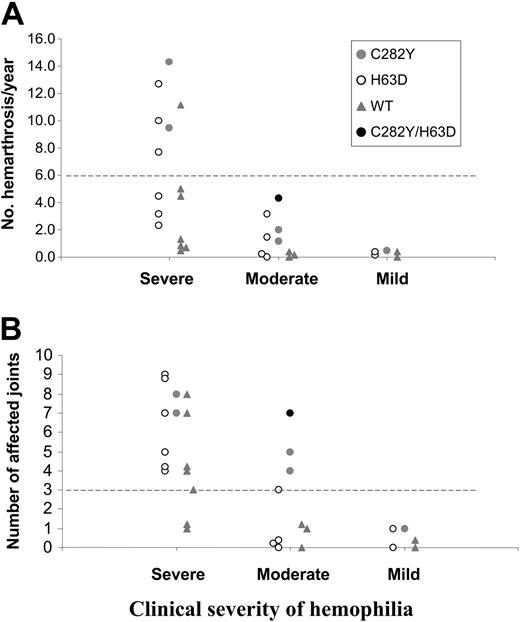
The patients were grouped according to severity of disease: severely affected (n = 17; factor VIII [FVIII]/IX ≤ 1%), moderately affected (n = 11; FVIII/IX > 1% and ≤ 5%), and mildly affected (n = 6; FVIII/IX > 5%). Arthropathy severity was arbitrarily defined as number of hemarthrosis/year and number of affected joints. No differences were seen between the ages of the patients in the 3 groups. The results are summarized in Figure 1.
Severity of hemophilic arthropathy in relation to HFE mutations. Number of episodes of hemarthrosis/year (A) and number of affected joints (B) according to clinical severity of hemophilia. Individual patients in each group are represented according to the presence of HFE mutations: C282Y heterozygous ( ), H63D carriers (○), compound heterozygote (•), and wild-type (WT) patients (
), H63D carriers (○), compound heterozygote (•), and wild-type (WT) patients ( ). Four patients were excluded: 2 with severe hemophilia (one doing prophylactic treatment at the time of the study and another with no clinical data available); 1 patient with moderate hemophilia without complete relevant clinical information; and 1 patient with mild disease doing immunotolerance treatment at the time of the study. The dotted lines represent the cutoff values established for hemarthrosis/year (> 6) as an indication of severity of the arthropathy. The cutoff for number of joints affected was arbitrarily established for this study (> 3).
). Four patients were excluded: 2 with severe hemophilia (one doing prophylactic treatment at the time of the study and another with no clinical data available); 1 patient with moderate hemophilia without complete relevant clinical information; and 1 patient with mild disease doing immunotolerance treatment at the time of the study. The dotted lines represent the cutoff values established for hemarthrosis/year (> 6) as an indication of severity of the arthropathy. The cutoff for number of joints affected was arbitrarily established for this study (> 3).
Severity of hemophilic arthropathy in relation to HFE mutations. Number of episodes of hemarthrosis/year (A) and number of affected joints (B) according to clinical severity of hemophilia. Individual patients in each group are represented according to the presence of HFE mutations: C282Y heterozygous ( ), H63D carriers (○), compound heterozygote (•), and wild-type (WT) patients (
), H63D carriers (○), compound heterozygote (•), and wild-type (WT) patients ( ). Four patients were excluded: 2 with severe hemophilia (one doing prophylactic treatment at the time of the study and another with no clinical data available); 1 patient with moderate hemophilia without complete relevant clinical information; and 1 patient with mild disease doing immunotolerance treatment at the time of the study. The dotted lines represent the cutoff values established for hemarthrosis/year (> 6) as an indication of severity of the arthropathy. The cutoff for number of joints affected was arbitrarily established for this study (> 3).
). Four patients were excluded: 2 with severe hemophilia (one doing prophylactic treatment at the time of the study and another with no clinical data available); 1 patient with moderate hemophilia without complete relevant clinical information; and 1 patient with mild disease doing immunotolerance treatment at the time of the study. The dotted lines represent the cutoff values established for hemarthrosis/year (> 6) as an indication of severity of the arthropathy. The cutoff for number of joints affected was arbitrarily established for this study (> 3).
Analysis of the impact of the HFE mutations on the number of hemarthrosis/year showed that 5 (63%) of 8 HFE-mutated patients in the severe group had more than 6 episodes in contrast with 1 (14%) of 7 wild-type patients (Figure 1A). In terms of number of affected joints, 100% (8/8) of HFE-mutated patients in the severe group had more than 3 joints affected as opposed to 4 (57%) of 7 wild-type patients (Figure 1B). The impact of HFE mutations was particularly visible in the moderate group where C282Y carriers are segregated from the other genotypes (Figure 1B). All C282Y carriers (n = 3) had more than 3 affected joints in contrast to the H63D single carriers or wild-type patients. The only compound heterozygous (in the moderate group) had the most severe signs of arthropathy in this group (Figure 1A-B). These results point to an impact of HFE mutations in hemophilic arthropathy compatible with a deleterious systemic effect of iron associated with deregulated iron absorption.
The results echo the results of an earlier study of the activation of the rat synovium by iron where a peak of the mitotic activity was seen among synovial cells at 8 hours after one single injection of ferric citrate sufficient to cause a transient increase above 100% in transferrin saturation.6 In conclusion, gene mutations that influence circulating iron levels and that in addition increase iron exit from macrophages7 should be considered among the genetic factors contributing to the heterogeneity of hemophilic arthropathy, as put forward by Abshire.2
Supported by the Forum Hematológico (Norte), the FCT, and the Gulbenkian Foundation.
We thank Susana Almeida for technical support in HFE genotyping.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal