Comment on Massullo et al, page 3397
The frustration of animal models aside, a simple cell culture model offers insight into how mutant neutrophil elastase causes hereditary neutropenia.
Neutrophil elastase constitutes the major hydrolytic enzyme of neutrophil granules, yet there had been little to suggest the extraordinarily malignant potential of this serine protease in hematopoiesis, heretofore known for its killing of pathogens and over-zealous activity in emphysematous lung disease. It thus proved surprising when it was found1 that heritable, heterozygous mutations of its gene, ELA2, cause cyclic hematopoiesis—an autosomal dominant disorder in which neutrophils and monocytes reciprocally oscillate in number with 3-week frequency—and are also the most common cause2 of the Kost-mann syndrome of severe congenital neutropenia (SCN), characterized by promyelocytic maturation arrest and evolution to myelodysplasia and acute myelogenous leukemia.
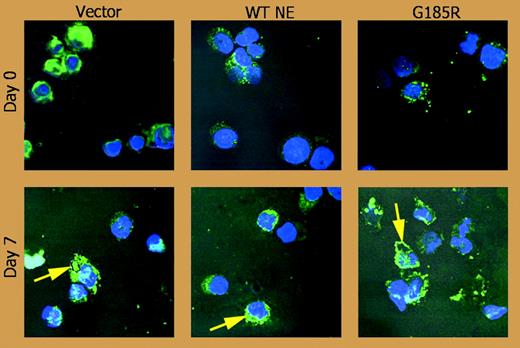
Ela2 knock-in mice are not neutropenic3 and the construction of transgenic mice has proven difficult, so Massullo and colleagues' retroviral expression of a particularly malevolent and strongly leukemia-associated SCN mutation,4 ELA2 G185, in promyelocytic cells affords the first model system in which to observe specific effects of pathologic forms of neutrophil elastase on differentiating cells. Their results are revealing. First, they find that the mutant neutrophil elastase provokes premature apoptosis of HL60 cells induced to differentiate with dimethyl sulfoxide (DMSO), resulting in fewer mature neutrophils and lending support to some prior observations. Second, they corroborate other findings of subcellular mislocalization of the mutant neutrophil elastase to membranes, where it may unleash untoward proteolytic activity. Third, expression of the mutant neutrophil elastase reduces the abundance of the adaptor protein 3 (AP3) complex. AP3 transports vacuolar cargo proteins out of the Golgi, and mutations of its beta subunit are the cause of human Hermansky-Pudlak syndrome 2 and canine cyclic neutropenia.5 It has been proposed that AP3 conveys neutrophil elastase to granules and that AP3's absence in the latter 2 diseases causes neutropenia by mislocalizing neutrophil elastase.5 Intriguingly, this new observation suggests an alternate possibility in which neutropenia could arise when mislocalized neutrophil elastase disrupts AP3, which could, in turn, more profoundly disturb granulopoiesis. ▪
Confocal immunofluorescence microscopy shows the subcellular distribution of NE (green) with counterstaining of nuclei (Hoechst, blue). See the complete figure in the article beginning on page 3397.
Confocal immunofluorescence microscopy shows the subcellular distribution of NE (green) with counterstaining of nuclei (Hoechst, blue). See the complete figure in the article beginning on page 3397.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal