Abstract
Interactions of endothelial cells with fibrin(ogen) are implicated in inflammation, angiogenesis, and wound healing. Cross-linking of the fibrinogen αC domains with factor XIIIa generates ordered αC oligomers mimicking polymeric arrangement of the αC domains in fibrin. These oligomers and those prepared with tissue transglutaminase were used to establish a mechanism of the αC domain–mediated interaction of fibrin with endothelial cells. Cell adhesion and chemical cross-linking experiments revealed that oligomerization of the αC domains by both transglutaminases significantly increases their RGD (arginyl–glycyl–aspartate)–dependent interaction with endothelial αVβ3 and to a lesser extent with αVβ5 and α5β1 integrins. The oligomerization promotes integrin clustering, thereby increasing cell adhesion, spreading, formation of prominent peripheral focal contacts, and integrin-mediated activation of focal adhesion kinase (FAK) and extracellular signal-regulated kinase (ERK) signaling pathways. The enhanced integrin clustering is likely caused by ordered juxtaposition of RGD-containing integrin-binding sites upon oligomerization of the αC domains and increased affinity of these domains for integrins. Our findings provide new insights into the mechanism of the αC domain–mediated interaction of endothelial cells with fibrin and imply its potential involvement in cell migration. They also suggest a new role for transglutaminases in regulation of integrin-mediated adhesion and signaling via covalent modification of integrin ligands.
Introduction
The plasma protein fibrinogen plays a prominent role in hemostasis and a number of other physiological and pathological processes. Vascular injury initiates platelet aggregation and blood coagulation, resulting in conversion of soluble fibrinogen into insoluble fibrin and formation of fibrin-rich blood clot. The clot plugs damaged vessel walls, preventing the loss of blood, and serves subsequently as a provisional matrix for cell adhesion, migration, and proliferation during wound healing and neovascularization.1 Particularly, interaction of leukocytes and endothelial cells with the fibrin clot modulates the inflammatory response and stimulates angiogenesis.2,3 This multitude of fibrin(ogen) functions is based on its ability to interact with numerous adhesion receptors present on the surface of leukocytes, endothelial cells, fibroblasts, platelets, and other cell types. Among these receptors, integrins represent a large class of heterodimeric transmembrane adhesion receptors that participate in a wide range of cell-matrix interactions.4 Fibrinogen contains multiple recognition sites for integrins, some of which appear cryptic and become exposed upon its conversion into fibrin.5-10
Fibrinogen is a complex multidomain protein consisting of 2 identical subunits, each composed of 3 polypeptide chains, Aα,Bβ, and γ.11,12 These chains are folded into a number of distinct domains grouped into several structural regions.13 The disulfide-linked NH2-terminal portions of all 6 chains form the central E region, while their COOH-terminal portions form 2 terminal D regions and 2 αC domains.11,12,14,15 The αC domains formed by the Aα chain residues 221 to 610 are located on the surface of the molecule and play an important role in modulation of various processes. They are involved in fibrin assembly,16,17 activation of factor XIII (FXIII),18 and modulation of fibrinolysis19,20 and cell adhesion via either bound fibronectin or their Aα572-574 arginyl–glycyl–aspartate (RGD) recognition motif.5,21,22 Previous studies showed that this motif is a predominant site for the interaction of fibrin(ogen) with endothelial cell integrin αVβ3.5 Another endothelial cell integrin, α5β1, was also found to interact with fibrin (ogen) in an RGD-dependent manner via this motif.7,23
According to the current view, in fibrinogen the αC domains interact intramolecularly with each other and with the central E region while in fibrin they switch to intermolecular interactions to form α polymers,24 which are covalently cross-linked by activated plasma transglutaminase factor XIIIa (FXIIIa).25 Tissue transglutaminase (tTG) also cross-links the αC domains in fibrin, although the cross-linking pattern seems to be different.26,27 Because fibrinogen is rather inert in the circulation while fibrin is highly reactive, the activity of the αC domains appears to be connected with their polymerization and cross-linking. This was confirmed in our recent study in which we found that the recombinant αC domains are able to form stable oligomers upon cross-linking with FXIIIa and that the adhesion of endothelial cells to the αC domains increased upon their oligomerization.28 The underlying mechanism for such effects remains unclear.
Our study also demonstrated that FXIIIa–cross-linked recombinant αC domain oligomers have an ordered structure and may adequately mimic the structure and properties of the αC domains in cross-linked fibrin.28 These oligomers, as well as the tTG–cross-linked αC oligomers, were used in the present study as models to clarify the mechanism(s) of the αC domain–mediated interaction of fibrin with endothelial cells. The experiments revealed that oligomerization and cross-linking of the αC domains by both transglutaminases significantly promote adhesion of endothelial cells via their integrin receptors, facilitate focal adhesion assembly via integrin clustering, and amplify integrin-mediated signaling.
Materials and methods
Proteins, antibodies, and recombinant fragments
Bovine α-thrombin and guinea pig liver tTG were from Sigma (St Louis, MO). Bovine serum albumin (BSA) and human FXIII were from Calbiochem (La Jolla, CA). The recombinant human fibrinogen αC domain including the Aα chain residues 221 to 610 was prepared as described earlier.29 A membrane-impermeable thiol-cleavable cross-linker 3,3′-dithiobis[sulfosuccinimidyl propionate] (DTSSP) was from Pierce (Rock-ford, IL). Purified integrins αVβ3, αVβ5, and α5β1; anti–β5 integrin polyclonal antibodies; and monoclonal antibodies (mAbs) P3G8 (anti-αV), LM609 and 23C6 (anti-αVβ3), P1F6 (anti-αVβ5), P1D6 (anti-α5β1), BHA2.1 (anti-α2β1), NKI-GoH3 (anti-α6β1), JB1A (anti-β1), and 25E11 (anti-β3) were obtained from Chemicon (Temecula, CA). Antiphosphotyrosine polyclonal antibodies were from BD Biosciences (San Diego, CA). Polyclonal antibodies to focal adhesion kinase (FAK) and phosphospecific antibodies to pTyr residues of FAK were from BioSource (Camarillo, CA). Polyclonal antibodies against extracellular signal-regulated kinase 1/2 (ERK1/2) and dually phosphorylated ERK1/2 were from Cell Signaling Technology (Beverly, MA). Monoclonal antibody 1D4 against an epitope located in the Aα349-406 region of the αC domain30 was a gift from Dr B. Kudryk (New York Blood Center).
Cell culture
Human umbilical vein endothelial cells (HUVECs) and Clonetics endothelial cell growth medium EBM-2 supplemented with EGM-2 SingleQuots were obtained from BioWhittaker (Walkersville, MD).
Cross-linking of the αC domains with factor XIIIa or tTG and purification of cross-linked oligomers
Cross-linking of the recombinant αC domain with FXIIIa and preparation of soluble cross-linked αC oligomers were performed as described earlier.19,28 Cross-linking of the αC domain with tTG was performed similarly to that with FXIIIa. The reaction mixture containing the αC domain at 1 mg/mL and tTG at 50 μg/mL in Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) with 10 mM Ca2+ was incubated for 2 hours at room temperature, centrifuged to remove nonsoluble material, and then fractionated by size-exclusion chromatography on a Superdex 200 HR column to prepare soluble cross-linked αC oligomers.
Solid-phase binding assays
Solid-phase binding was performed in microtiter plates using enzyme-linked immunosorbent assay (ELISA). Microtiter plate wells (Fisher Scientific, Hampton, NH) were coated overnight with 100 μL per well monomeric αC domains (αC monomers) or αC oligomers cross-linked by either factor XIIIa (αC(FXIII) oligomers) or tissue transglutaminase (αC(tTG) oligomers), all at 20 μg/mL in 0.1 M Na2CO3, pH 9.5 (coating buffer). The wells were then blocked with 1% BSA in TBS. The amounts of αC monomers and oligomers adsorbed to microtiter wells were the same as revealed by ELISA with mAb 1D4. After washing with TBS containing 0.05% Triton X-100, 1 mM MgCl2, and 1 mM MnCl2, the αVβ3 integrin in the same buffer was added to the wells at 20 μg/mL and incubated for 1 hour. Bound αVβ3 was measured by reaction with mAb P3G8 and peroxidase-conjugated antimouse polyclonal antibodies. A TMB Microwell peroxide substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was added to the wells, and the amount of bound ligand was measured spectrophotometrically at 450 nm.
Transmission electron microscopy studies
Samples for electron microscopy were prepared by spraying the αC monomer or αC oligomers, both at 40 μg/mL in a volatile buffer (50 mM ammonium formate, pH 7.4, or 0.125% acetic acid, pH 3.5) and 25% to 30% glycerol, onto freshly cleaved mica and rotary shadowing with tungsten in a vacuum evaporator as previously described.31 Specimens were examined in a Philips 400 electron microscope (Philips Electronic Instruments, Hillsboro, OR) at 80 kV and × 60 000 magnification.
Cell adhesion assays
Twenty-four–well tissue culture plastic plates (Midwest Scientific, St. Louis, MO) were coated with αC monomers, αC(FXIII) oligomers, or αC(tTG) oligomers, all at 20 μg/mL in TBS, for 16 hours at 4°C, and then blocked with 10 mg/mL BSA. The amounts of αC monomers and oligomers adsorbed to tissue culture plastic wells were the same as revealed by ELISA with mAb 1D4.
For adhesion experiments, HUVECs were labeled overnight with 20 μCi (0.74 MBq)/mL Tran35S-Label (MP Biomedicals, Irvine, CA). After detachment by trypsinization and blocking excess of trypsin with 0.5 mg/mL soybean trypsin inhibitor, 5 × 104 35S-labeled HUVECs were plated into each well in serum-free Dulbecco modified Eagle medium (DMEM) (Invitrogen, Carlsbad, CA) containing 5 mg/mL BSA and allowed to adhere for 20 minutes at 37°C. In some experiments, cells were plated in the presence of 250 μg/mL GRGDSP or GRGESP peptides (American Peptide, Sunnyvale, CA). To study the role of individual integrins in HUVEC adhesion to αC monomers and oligomers, the 35S-labeled cells were preincubated for 30 minutes on ice with 20 μg/mL function-blocking anti-αVβ3 mAb LM609, anti-αVβ5 mAb P1F6, and anti-α5β1 mAb P1D6 before plating on the substrates in the presence of the antibodies. Adherent cells were washed 3 times with phosphate-buffered saline (PBS) and lysed in 1% sodium dodecyl sulfate (SDS). The bound radioactivity was counted in a Beckman LS 3801 scintillation counter (Beckman Coulter, Fullerton, CA) and converted into the number of adherent cells by referring to the levels of 35S incorporation per 103 cells.
Cell spreading assays and quantitation of cell area on substrates
A total of 2 × 104 unlabeled HUVECs were plated in serum-free DMEM with 5 mg/mL BSA at 37°C for indicated periods of time on glass coverslips coated with 20 μg/mL αC monomers, αC(FXIII) oligomers, or αC(tTG) oligomers. At different time points of spreading, cells were fixed with 3.7% paraformaldehyde, stained with Coomassie blue, destained, and photographed. The outlines and cell areas of 100 randomly chosen nonadjacent cells were analyzed using Image-Pro Plus microscopy software (Media Cybernetics, Baltimore, MD) calibrated with an Applied Micro Stage micrometer (EF Precision Group, Willow Grove, PA).
Immunofluorescence
Glass coverslips were coated with 20 μg/mL αC monomers, αC(FXIII) oligomers, or αC(tTG) oligomers and then blocked with 10 mg/mL BSA. Serum-starved HUVECs were trypsinized and then plated in DMEM with 10 mg/mL albumin on the αC domain monomers or oligomers. After 2 hours the cells were fixed with 3% paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS. The cells were double stained with 20 μg/mL mAb 23C6 against αVβ3 and 10 μg/mL rabbit polyclonal antiphosphotyrosine antibodies, followed by rhodamine-conjugated anti–mouse and fluorescein-labeled anti–rabbit immunoglobulin G (IgG). Cells were photographed using a Nikon Eclipse E800 microscope (Nikon, Melville, NY) with a 60×/1.4 objective and Spot RT digital camera. Images were acquired with Advance Spot software (Diagnostic Instruments).
Quantitation of ligand-bound integrins by reversible chemical cross-linking to substrates
A total of 107 HUVECs were plated in serum-free DMEM with 10 mg/mL BSA on T150 tissue culture flasks coated with 20 μg/mL αC monomers, αC(FXIII) oligomers, or αC(tTG) oligomers. Two hours later cells were washed with PBS and chemically cross-linked to substrates with 2 mM DTSSP in PBS for 30 minutes at 4°C. To stop the cross-linking, the cells were incubated with TBS for 10 minutes at 4°C. Then they were extracted 4 times for 20 minutes with 25 mL 0.1% SDS in H2O containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM benzamidine, 10 μg/mL leupeptin, 10 μg/mL aprotinin). Recovery of the cross-linked material (de–cross-linking) was performed on an orbital shaker at 40°C for 1 hour with 12 mL TBS containing 100 mM dithiothreitol (DTT), 0.1% SDS, and 5 μg/mL BSA. To account for a difference in the number of adherent cells on the substrates, all 3 fractions were normalized to represent 2 × 106 adherent cells. The recovered proteins were concentrated in Amicon Ultra-4 concentrators (Millipore, Bedford, MA) and then precipitated with ice-cold acetone. Integrins in the recovered protein fractions were analyzed by 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with antibodies against selected integrin subunits; 10 ng purified αVβ3, α5β1, and αVβ5 integrins were used as external standards on the blots. Blots were developed with SuperSignal West Pico Substrate (Pierce). The developed blots were subjected to densitometry using NIH Image 1.63f software. A calibration with different integrin loadings showed a linear increase in signal within the range of 0.5 to 25 ng. The amounts of cellular αVβ3, α5β1, and αVβ5 integrins chemically cross-linked to the 3 substrates were compared with purified integrin standards by densitometry and expressed relative to those in HUVECs adherent on αC monomers. Using this method, a total number of integrin receptors per cell was estimated as follows: (2.1 ± 0.2) × 105; (2.3 ± 0.2) × 105; and (1.6 ± 0.2) × 105 for αVβ3, α5β1, and αVβ5, respectively.
Analysis of adhesion-mediated phosphorylation of FAK and ERK1/2
Analysis of adhesion-mediated FAK and ERK1/2 phosphorylation was performed as described previously.32-34 A total of 106 serum-starved HUVECs in serum-free DMEM with 10 mg/mL BSA were kept in suspension or plated on tissue culture plates coated with 20 μg/mL αC monomers or αC(FXIII) oligomers. Two hours later the adherent cells were washed with PBS and lysed in ice-cold buffer (20 mM Tris; pH 7.4, 100 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM EGTA [ethyleneglycoltetraacetic acid], 20 mM Na4P2O7, 1 mM NaF, 1% Triton X-100, 0.1% SDS) containing 2 mM Na3VO4 and protease inhibitors (1 mM PMSF, 1 mM benzamidine, 10 μg/mL leupeptin, 10 μg/mL aprotinin). Protein concentrations were determined with BCA protein Assay Kit (Pierce). A total of 200 μg of total cell extracts was subjected to immunoprecipitation with anti-FAK antibodies. The resulting immune complexes and total cell lysates were separated on 8% SDS-PAGE gels and analyzed by immunoblotting with antibodies to FAK, phosphotyrosine, and phosphospecific antibodies against selected pTyr residues of FAK. Blots were developed with SuperSignal West Pico Substrate and scanned by densitometer with NIH Image 1.63f software. The extent of FAK phosphorylation was normalized to the amounts of total FAK in each sample and expressed relative to that in HUVECs kept in suspension.
Alternatively, the total cell extracts were also separated on 15% SDS-PAGE gels and examined by immunoblotting with antibodies to ERK1/2 and dually phosphorylated (activated) ERK1/2. ERK1/2 bands visualized by ECL chemiluminescence with West Pico Substrate were scanned and digitized by NIH Image 1.63f software. The levels of ERK1 and ERK2 phosphorylation were normalized to the amounts of total ERK1 and ERK2 in each sample and expressed relative to those in HUVECs in suspension.
Results
Characterization of αC oligomers generated by cross-linking with transglutaminases
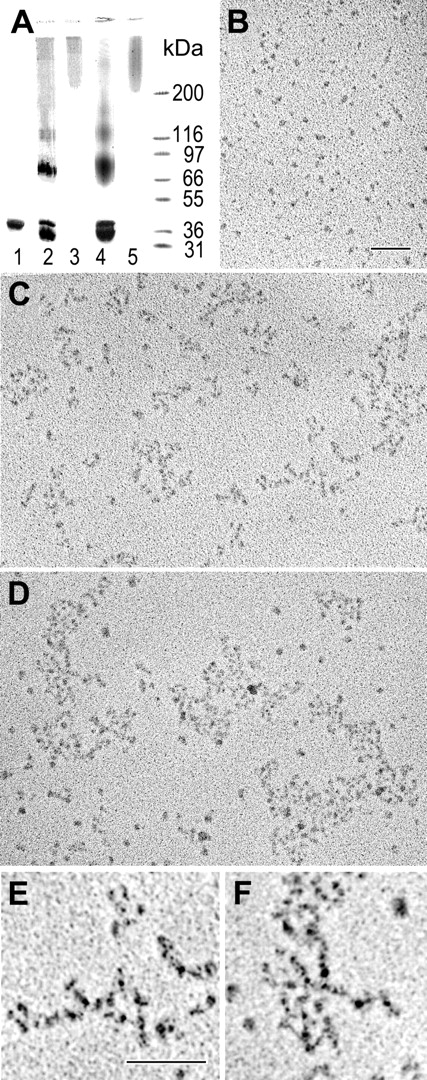
We demonstrated recently that treatment of the recombinant αC domains with factor XIIIa results in cross-linked αC oligomers, αC(FXIII), which are soluble, stable, and have an ordered structure.28 In the current study, we prepared and characterized αC oligomers cross-linked with guinea pig liver tissue transglutaminase, αC(tTG). SDS-PAGE analysis revealed that, similarly to cross-linking with factor XIIIa, treatment of the αC domain with tTG led to the formation of covalently cross-linked material with the mobility expected for dimers, trimers, and higher order oligomers (Figure 1A). The fraction containing soluble oligomers with molecular mass higher than 200 kDa was separated by size-exclusion chromatography and analyzed by electron microscopy after preparation by the method of rotary shadowing. The analysis revealed that these oligomers were similar to those prepared with factor XIIIa (Figure 1B-F). In both cases, the oligomers consisted of branched or bundled strands built of 4 nm globular structures. These oligomers seemed to be regular in structure, with strands that were about 8 nm or 2 monomers in width, but they were often branched and quite complex. Some very large polymers were observed in these preparations. Overall, cross-linking of the αC domains with both transglutaminases produced αC oligomers with similar regular structure. Both types of oligomers, αC(FXIII) and αC(tTG), were further used in experiments with endothelial cells.
Preparation and characterization of αC(FXIII) and αC(tTG) oligomers. (A) SDS-PAGE analysis of the recombinant αC domain fragment and its oligomers. The αC domain (lane 1) was covalently cross-linked with factor XIIIa (lane 2) or tTG (lane 4), and the resulting material was subjected to size-exclusion chromatography to separate high molecular mass fractions containing αC(FXIII) oligomers (lane 3) or αC(tTG) oligomers (lane 5); the right outer lane contains protein markers of the indicated molecular mass. (B-D) Electron microscopy of the rotary shadowed samples of the αC domain fragment (B) and its αC(FXIII) and αC(tTG) oligomers (C-D, respectively). Bar indicates 100 nm. (E-F) Higher magnification and contrast images of small portions of panels C and D, respectively, showing details of the branching polymers. Bar indicates 100 nm.
Preparation and characterization of αC(FXIII) and αC(tTG) oligomers. (A) SDS-PAGE analysis of the recombinant αC domain fragment and its oligomers. The αC domain (lane 1) was covalently cross-linked with factor XIIIa (lane 2) or tTG (lane 4), and the resulting material was subjected to size-exclusion chromatography to separate high molecular mass fractions containing αC(FXIII) oligomers (lane 3) or αC(tTG) oligomers (lane 5); the right outer lane contains protein markers of the indicated molecular mass. (B-D) Electron microscopy of the rotary shadowed samples of the αC domain fragment (B) and its αC(FXIII) and αC(tTG) oligomers (C-D, respectively). Bar indicates 100 nm. (E-F) Higher magnification and contrast images of small portions of panels C and D, respectively, showing details of the branching polymers. Bar indicates 100 nm.
Oligomerization of the αC domains promotes RGD-dependent adhesion of endothelial cells via αVβ3, αVβ5, and α5β1 integrins
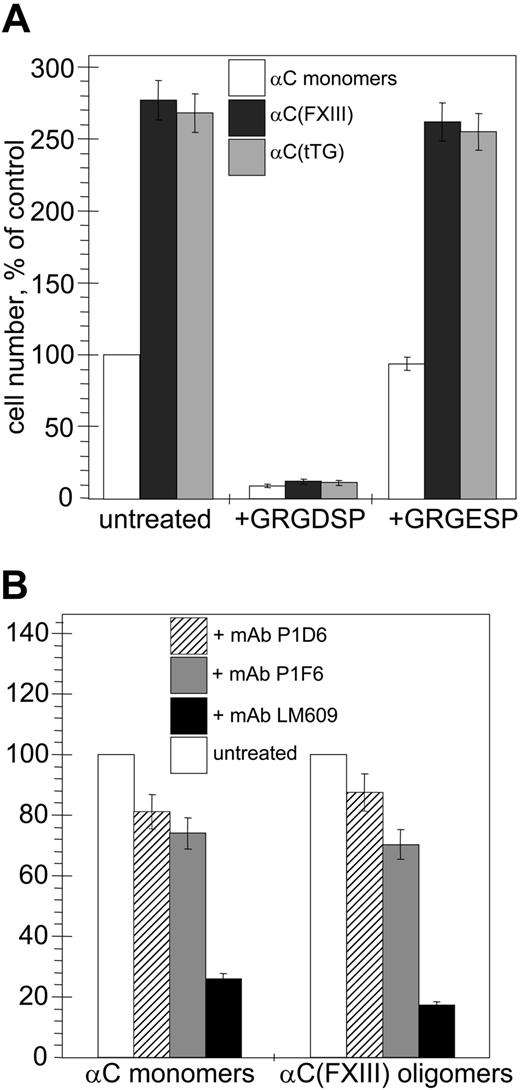
First, we compared adhesion of human umbilical vein endothelial cells (HUVECs) to monomeric recombinant αC domains and αC(tTG) or αC(FXIII) oligomers in short-term static cell adhesion assays (Figure 2A). In both cases, HUVEC adhesion to αC oligomers was about 3-fold higher than that to αC monomers. This indicates that oligomerization of αC domains increases their adhesive capacity for endothelial cells and that the cell-binding properties of tTG- and FXIIA–cross-linked αC oligomers are similar. The adhesion to all species was abolished by treatment of the cells with GRGDSP peptide, while it remained unaffected by the control scrambled GRGESP peptide, in agreement with the previous findings that this interaction is RGD dependent.
Oligomerization of the αC domains stimulates RGD-dependent adhesion of endothelial cells via αVβ3, αVβ5, and α5β1 integrins. Quantitative adhesion assays were performed with 5 × 104 HUVECs plated in serum-free DMEM for 20 minutes at 37°C on plastic wells coated with 20 μg/mL αC monomers, αC(FXIII) oligomers, or αC(tTG) oligomers. (A) Effects of oligomerization and RGD-containing peptide on cell adhesion to the αC domain species (□, αC monomers; ▪, αC(FXIII); ▦, αC(tTG)). Cells were plated without treatment or in the presence of 250 μg/mL GRGDSP or control GRGESP peptides. The number of untreated adherent cells on αC monomers was taken as 100%. (B) The role of individual integrins in adhesion to αC monomers and oligomers. Cells were plated without treatment or in the presence of 20 μg/mL blocking antibodies to α5β1 (mAb P1D6; ▨), αVβ5 (mAb P1F6; ▦), or αVβ3 (mAb LM609; ▪). The numbers of untreated cells (□) adherent to αC monomers or αC(FXIII) oligomers were taken as 100%. The results shown in both panels are the means ± standard deviations of 3 independent experiments performed in duplicates.
Oligomerization of the αC domains stimulates RGD-dependent adhesion of endothelial cells via αVβ3, αVβ5, and α5β1 integrins. Quantitative adhesion assays were performed with 5 × 104 HUVECs plated in serum-free DMEM for 20 minutes at 37°C on plastic wells coated with 20 μg/mL αC monomers, αC(FXIII) oligomers, or αC(tTG) oligomers. (A) Effects of oligomerization and RGD-containing peptide on cell adhesion to the αC domain species (□, αC monomers; ▪, αC(FXIII); ▦, αC(tTG)). Cells were plated without treatment or in the presence of 250 μg/mL GRGDSP or control GRGESP peptides. The number of untreated adherent cells on αC monomers was taken as 100%. (B) The role of individual integrins in adhesion to αC monomers and oligomers. Cells were plated without treatment or in the presence of 20 μg/mL blocking antibodies to α5β1 (mAb P1D6; ▨), αVβ5 (mAb P1F6; ▦), or αVβ3 (mAb LM609; ▪). The numbers of untreated cells (□) adherent to αC monomers or αC(FXIII) oligomers were taken as 100%. The results shown in both panels are the means ± standard deviations of 3 independent experiments performed in duplicates.
HUVECs express at least 3 integrins, αVβ3, αVβ5, and α5β1, which interact with their ligands in an RGD-dependent manner. Therefore, we examined the roles of these integrins in adhesion of HUVECs to αC monomers and αC(FXIII) oligomers using function-blocking antibodies (Figure 2B). Treatment of the cells with mAbs P1D6 and P1F6 against, respectively, the α5β1 and αVβ5 integrins, moderately decreased (by about 15% to 30%) adhesion of HUVECs to αC monomers and αC(FXIII) oligomers. In contrast, the use of anti-αVβ3 mAb LM609 sharply reduced (by about 75% to 85%) adhesion of HUVECs to both the monomeric and oligomeric αC domains. In control experiments with function-blocking mAbs, we found no involvement of RGD-independent α2β1 and α6β1 integrins expressed on HUVECs in adhesion of these cells to the αC domain species (data not shown). Thus, formation of covalently cross-linked αC domain oligomers by either FXIIA or tTG strongly stimulates RGD-dependent adhesion primarily via the αVβ3 integrin and to a lesser extent via the α5β1 and αVβ5 integrins.
Oligomerization of the αC domains enhances endothelial cell spreading and facilitates focal adhesion assembly
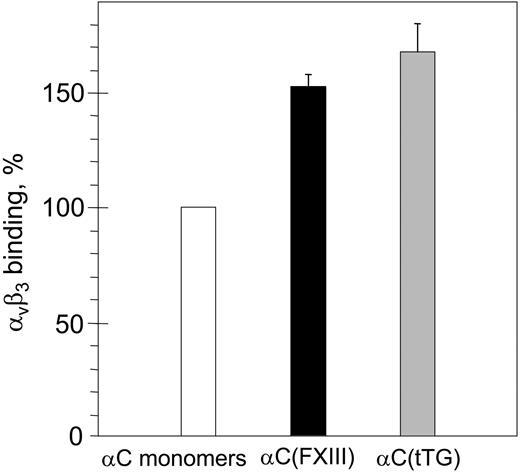
To clarify the relationship between the oligomerization and the increased adhesion, we first tested binding of purified αVβ3 integrin to immobilized αC monomers and αC(FXIII) or αC(tTG) oligomers by ELISA (Figure 3). While no difference in the amounts of αC monomers and oligomers immobilized on microtiter plastic wells was detected with anti–αC domain mAb 1D4 (data not shown), a moderate increase (1.5-fold to 1.7-fold) in binding of αVβ3 to both types of αC oligomers was observed. Although such increased binding could be a result of an increased affinity of RGD-containing binding sites in the oligomers, it cannot itself account for the dramatic increase in the adhesive capacity of the oligomers. Therefore, we focused on morphological changes and localization of integrins in HUVECs adherent to the αC monomers and oligomers.
Binding of purified αVβ3 integrin to immobilized αC monomers and αC(FXIII) or αC(tTG) oligomers. Plastic wells were coated with 20 μg/mL αC monomers (□), αC(FXIII) oligomers (▪), or αC(tTG) oligomers (▦). Binding of purified αVβ3 integrin (20 μg/mL) was measured by ELISA with anti-β3 mAb 25E11 followed by secondary goat anti–mouse IgG coupled with peroxidase. Binding to αC monomers was taken as 100%. Results are the means ± standard deviations of 2 independent experiments performed in triplicates.
Binding of purified αVβ3 integrin to immobilized αC monomers and αC(FXIII) or αC(tTG) oligomers. Plastic wells were coated with 20 μg/mL αC monomers (□), αC(FXIII) oligomers (▪), or αC(tTG) oligomers (▦). Binding of purified αVβ3 integrin (20 μg/mL) was measured by ELISA with anti-β3 mAb 25E11 followed by secondary goat anti–mouse IgG coupled with peroxidase. Binding to αC monomers was taken as 100%. Results are the means ± standard deviations of 2 independent experiments performed in triplicates.
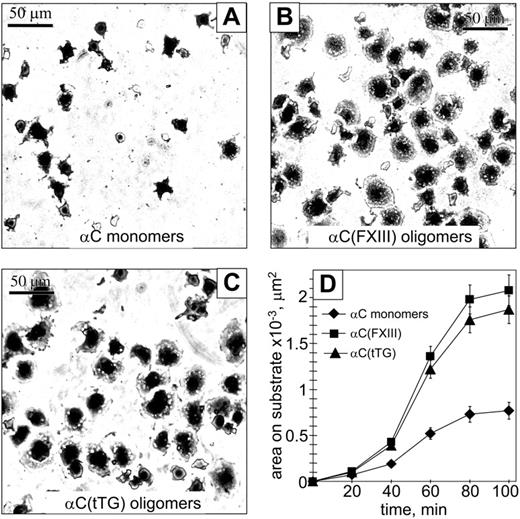
We next evaluated whether oligomerization of the αC domains affects cell spreading and focal adhesion formation. Ninety minutes after plating on substrates in serum-free medium, HUVECs appeared significantly more spread on the αC(FXIII) and αC(tTG) oligomers than on the αC monomers (Figure 4A-C). Quantification of the average spreading areas showed a time-dependent increase for all 3 substrates; however, HUVECs displayed a greater increase of the average spreading area when adherent to the αC(FXIII) and αC(tTG) oligomers (Figure 4D).
Oligomerization of the αC domains facilitates endothelial cell spreading. A total of 2 × 104 HUVECs in serum-free DMEM were plated at 37°C for indicated periods of time on plastic wells coated with 20 μg/mL αC monomers (A), αC(FXIII) oligomers (B), or αC(tTG) oligomers (C). At different time points of spreading, cells were fixed with 3.7% paraformaldehyde, stained with Coomassie blue, destained, and photographed. Shown are representative photographs of cells 90 minutes after plating on the substrates. Bar = 50 μm. (D) Time-dependent increase in cell spreading on αC monomers ( ) and oligomers (▪, αC(FXIII); ▴, αC(tTG)). The average areas were determined for 120 sparsely plated cells on each substrate. Results are the means ± standard deviations of 2 independent experiments performed in triplicate.
) and oligomers (▪, αC(FXIII); ▴, αC(tTG)). The average areas were determined for 120 sparsely plated cells on each substrate. Results are the means ± standard deviations of 2 independent experiments performed in triplicate.
Oligomerization of the αC domains facilitates endothelial cell spreading. A total of 2 × 104 HUVECs in serum-free DMEM were plated at 37°C for indicated periods of time on plastic wells coated with 20 μg/mL αC monomers (A), αC(FXIII) oligomers (B), or αC(tTG) oligomers (C). At different time points of spreading, cells were fixed with 3.7% paraformaldehyde, stained with Coomassie blue, destained, and photographed. Shown are representative photographs of cells 90 minutes after plating on the substrates. Bar = 50 μm. (D) Time-dependent increase in cell spreading on αC monomers ( ) and oligomers (▪, αC(FXIII); ▴, αC(tTG)). The average areas were determined for 120 sparsely plated cells on each substrate. Results are the means ± standard deviations of 2 independent experiments performed in triplicate.
) and oligomers (▪, αC(FXIII); ▴, αC(tTG)). The average areas were determined for 120 sparsely plated cells on each substrate. Results are the means ± standard deviations of 2 independent experiments performed in triplicate.
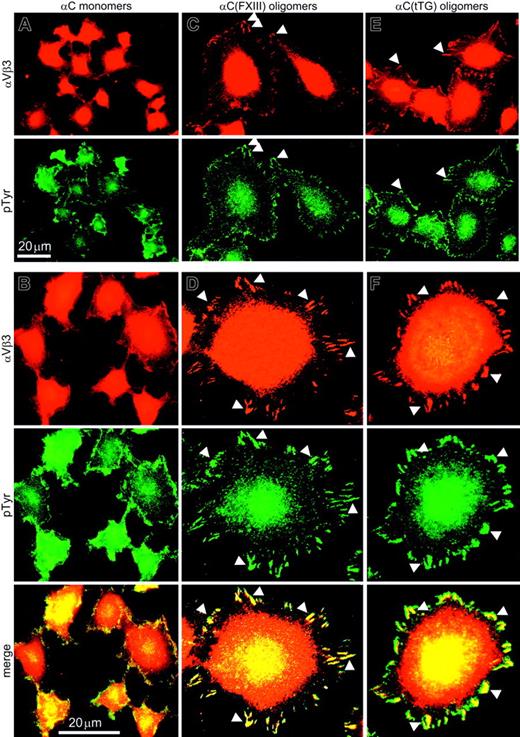
To assess the state of adhesion complexes in HUVECs adherent to the αC domain monomers and oligomers, immunostaining for the αVβ3 integrin and phosphotyrosine was performed and 120 sparsely plated cells on each substrate were examined. In HUVECs plated on αC monomers, αVβ3 and phosphotyrosine were uniformly distributed over the cell surface with occasional punctate staining along the cell periphery (Figure 5A). Analysis at higher magnification revealed few peripheral focal complexes but no mature focal adhesions in the case of the monomeric substrate (Figure 5B). In contrast, a distinctive accumulation of αVβ3 and phosphotyrosine along the cell edges was visible in HUVECs adherent to αC(FXIII) and αC(tTG) oligomers (Figure 5C,E). At higher magnification, well-developed peripheral focal adhesions exceeding 2 μm in length were visualized in at least 80% of HUVECs on either αC(FXIII) or αC(tTG) oligomers (Figure 5D,F), whereas less than 5% of cells on αC monomers displayed prominent focal contacts (Figure 5B). These results indicate that oligomerization of the αC domains promotes clustering of cell surface integrins as judged by a significant increase in the size of adhesion complexes.
Endothelial cells assemble prominent focal adhesions on αC(FXIII) and αC(tTG) oligomers but not on αC monomers. HUVECs in serum-free DMEM were plated on 20 μg/mL αC monomers (A-B), αC(FXIII) oligomers (C-D), or αC(tTG) oligomers (E-F) for 2 hours. Paraformaldehyde-fixed, Triton X-100–permeabilized cells were double stained with anti-β3 integrin mAb 25E11 and polyclonal antiphosphotyrosine antibodies, followed by rhodamine-labeled anti–mouse and fluorescein-conjugated anti–rabbit IgG. A clear peripheral staining for αVβ3 integrin and phosphotyrosine was observed at lower magnification in HUVECs on αC oligomers (C,E) but not on monomeric αC domains (A). At higher magnification, well-developed focal contacts containing αVβ3 integrin and phosphotyrosine were detected in HUVECs on αC oligomers (D,F), whereas no distinct focal adhesions were formed on αC monomers (B). Bars indicate 20 μm. Arrowheads mark colocalization of αVβ3 integrin and phosphotyrosine in the peripheral focal contacts of HUVECs on αC oligomers.
Endothelial cells assemble prominent focal adhesions on αC(FXIII) and αC(tTG) oligomers but not on αC monomers. HUVECs in serum-free DMEM were plated on 20 μg/mL αC monomers (A-B), αC(FXIII) oligomers (C-D), or αC(tTG) oligomers (E-F) for 2 hours. Paraformaldehyde-fixed, Triton X-100–permeabilized cells were double stained with anti-β3 integrin mAb 25E11 and polyclonal antiphosphotyrosine antibodies, followed by rhodamine-labeled anti–mouse and fluorescein-conjugated anti–rabbit IgG. A clear peripheral staining for αVβ3 integrin and phosphotyrosine was observed at lower magnification in HUVECs on αC oligomers (C,E) but not on monomeric αC domains (A). At higher magnification, well-developed focal contacts containing αVβ3 integrin and phosphotyrosine were detected in HUVECs on αC oligomers (D,F), whereas no distinct focal adhesions were formed on αC monomers (B). Bars indicate 20 μm. Arrowheads mark colocalization of αVβ3 integrin and phosphotyrosine in the peripheral focal contacts of HUVECs on αC oligomers.
Oligomerization of the αC domains increases the amounts of ligand-bound αVβ3, αVβ5, and α5β1 integrins
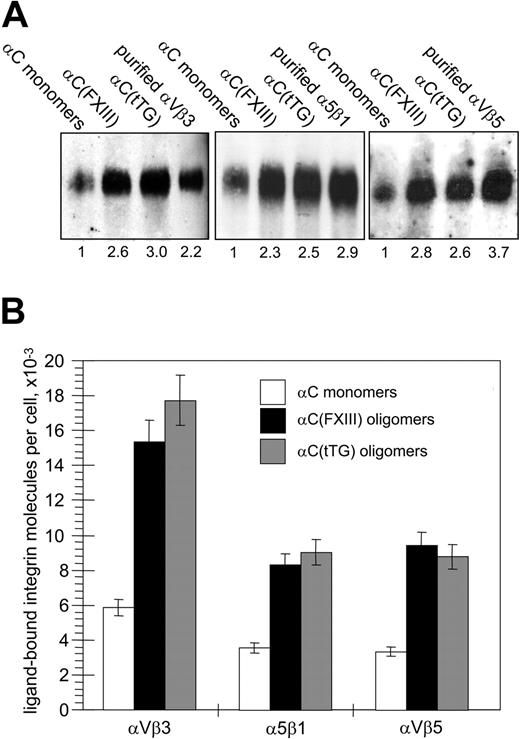
Given the enhancement of cell adhesion and assembly of large integrin clusters, we expected an increase in ligand-bound integrins for HUVECs adherent on αC oligomers. Integrin-ligand binding is a prerequisite for chemical cross-linking of integrins to their ligands, while the amounts of chemically cross-linked integrins are proportional to the number of integrin-ligand bonds and overall adhesion strength.34,35 A reversible cell-impermeable cross-linker DTSSP with a short (1.3 nm [13 Å]) spacer arm was used to compare the amounts of ligand-bound integrins on HUVECs adherent to the αC-domain monomers and oligomers. In these experiments, a relatively small proportion of cellular pools of αVβ3, αVβ5, and α5β1 integrins (1.5% to 3%) was found to be cross-linked to immobilized αC monomers, whereas the amounts of cross-linked integrins were increased in the cells attached to the αC oligomers (Figure 6A). Quantitative immunoblotting for the β3, β1, and β5 integrin subunits and normalization for the number of adherent cells revealed a significant (about 2.3-fold to 3.0-fold) increase in the amounts of cross-linked integrins in HUVECs on the αC(FXIII) and αC(tTG) oligomers (Figure 6B). Hence, transglutaminase-mediated oligomerization of the αC domains stimulates their ability to bind αVβ3, αVβ5, and α5β1 integrins.
Oligomerization of the αC domains increases the amounts of αVβ3, αVβ5, and α5β1 integrins chemically cross-linked to substrate. (A) HUVECs in serum-free DMEM were plated for 2 hours on 20 μg/mL αC monomers, αC(FXIII) oligomers, or αC(tTG) oligomers; 5 × 106 adherent cells were cross-linked to the substrates with 2 mM DTSSP in PBS for 30 minutes at 4°C and extracted 4 times for 20 minutes with 0.1% SDS. The cross-linked material was recovered by treating plates for 1 hour at 37°C with a buffer containing 100 mM DTT and 0.1% SDS and then concentrated and analyzed by SDS-PAGE and immunoblotting for the β3, β1, and β5 integrin subunits. Right lanes on the gels contained 10 ng purified αVβ3, αVβ5, and α5β1 integrins. Shown is a representative of 3 experiments. Numbers below the blots refer to the relative amounts of individual integrins, as determined by densitometry and normalized to their amounts cross-linked to αC monomers. (B) Bands corresponding to cellular integrins in panelAwere compared with external standards of purified integrins and then converted to the numbers of ligand-bound integrin receptors per cell. □ indicates αC monomers; ▪, αC(FXIII) oligomers; and ▦, αC(tTG) oligomers. Results are the means ± standard deviations of 3 independent experiments.
Oligomerization of the αC domains increases the amounts of αVβ3, αVβ5, and α5β1 integrins chemically cross-linked to substrate. (A) HUVECs in serum-free DMEM were plated for 2 hours on 20 μg/mL αC monomers, αC(FXIII) oligomers, or αC(tTG) oligomers; 5 × 106 adherent cells were cross-linked to the substrates with 2 mM DTSSP in PBS for 30 minutes at 4°C and extracted 4 times for 20 minutes with 0.1% SDS. The cross-linked material was recovered by treating plates for 1 hour at 37°C with a buffer containing 100 mM DTT and 0.1% SDS and then concentrated and analyzed by SDS-PAGE and immunoblotting for the β3, β1, and β5 integrin subunits. Right lanes on the gels contained 10 ng purified αVβ3, αVβ5, and α5β1 integrins. Shown is a representative of 3 experiments. Numbers below the blots refer to the relative amounts of individual integrins, as determined by densitometry and normalized to their amounts cross-linked to αC monomers. (B) Bands corresponding to cellular integrins in panelAwere compared with external standards of purified integrins and then converted to the numbers of ligand-bound integrin receptors per cell. □ indicates αC monomers; ▪, αC(FXIII) oligomers; and ▦, αC(tTG) oligomers. Results are the means ± standard deviations of 3 independent experiments.
Oligomerization of the αC domains amplifies integrin-mediated signaling to FAK and ERK
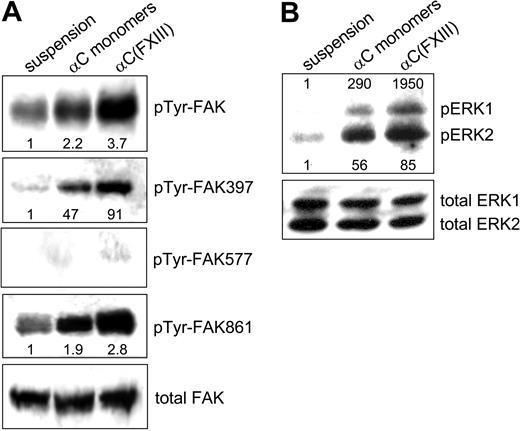
Integrins serve as signal transduction receptors, which require both clustering and ligand occupancy for a full biologic response.4 Because transglutaminase-mediated oligomerization of the αC domains both increases integrin binding and promotes clustering of these receptors, we set out to test the effects of such oligomerization on integrin-dependent signal transduction (Figure 7). Activation of FAK upon integrin engagement is a critical early step in integrin signaling that is involved in many aspects of cell behavior.36 Up to 6 tyrosine residues of FAK can be phosphorylated upon integrin-mediated cell-matrix adhesion.37 In the present study, antiphosphotyrosine and phosphospecific polyclonal antibodies to pTyr-FAK397, pTyr-FAK577, and pTyr-FAK861 were employed to evaluate the cellular response to adhesion on the αC monomers and cross-linked αC(FXIII) oligomers. Compared with HUVECs in suspension, adhesion to either αC monomers or αC(FXIII) oligomers raised the overall level of FAK phosphorylation, with a more robust increase observed on the oligomers (Figure 7A). Phosphorylation of Tyr397 in FAK, which reports the level of integrin tethering proportional to the number of integrin-ligand bonds,34 was markedly enhanced by adhesion to both substrates. Yet again, the increase appeared 2 times higher for HUVECs plated on the αC(FXIII) oligomers compared with αC monomers. Very little if any phosphorylation of Tyr577 residue of FAK was observed in nonadherent and adherent HUVECs. Phosphorylation of FAK residue Tyr861, which occurs in response to integrin clustering but does not require ligand binding by integrins,34 was induced about 2-fold by plating cells on the αC monomers and almost 3-fold by adhesion to the αC(FXIII) oligomers. No difference in adhesion-mediated signaling was observed between the αC(tTG) and αC(FXIII) oligomers (data not shown). Together, these results indicate that transglutaminase-mediated oligomerization of the αC domains amplifies adhesion-dependent phosphorylation of FAK due to up-regulation of both integrin binding and clustering.
Oligomerization of the αC domains amplifies adhesion-dependent activation of FAK and ERK. HUVECs were either kept in suspension or plated in serum-free DMEM for 2 hours on tissue culture plates coated with 20 μg/mL αC monomers or αC(FXIII) oligomers. (A) Phosphorylation of FAK tyrosines 397, 577, and 861 was examined by SDS-PAGE and immunoblotting with specific polyclonal antibodies (see “Materials and methods”). Overall tyrosine phosphorylation of FAK was tested by immunoprecipitation of FAK, followed by SDS-PAGE and immunoblotting with polyclonal antiphosphotyrosine antibodies. (B) Adhesion-dependent phosphorylation of ERK was analyzed by immunoblotting with antibodies against dually phosphorylated ERK1/2 and total ERK1/2. Panels A and B are representative of 3 independent experiments for FAK and ERK.
Oligomerization of the αC domains amplifies adhesion-dependent activation of FAK and ERK. HUVECs were either kept in suspension or plated in serum-free DMEM for 2 hours on tissue culture plates coated with 20 μg/mL αC monomers or αC(FXIII) oligomers. (A) Phosphorylation of FAK tyrosines 397, 577, and 861 was examined by SDS-PAGE and immunoblotting with specific polyclonal antibodies (see “Materials and methods”). Overall tyrosine phosphorylation of FAK was tested by immunoprecipitation of FAK, followed by SDS-PAGE and immunoblotting with polyclonal antiphosphotyrosine antibodies. (B) Adhesion-dependent phosphorylation of ERK was analyzed by immunoblotting with antibodies against dually phosphorylated ERK1/2 and total ERK1/2. Panels A and B are representative of 3 independent experiments for FAK and ERK.
We also examined integrin-dependent activation of ERK in HUVECs plated on αC monomers and oligomers (Figure 7B). Immunoblotting with phosphospecific antibodies revealed that adhesion to both substrates sharply increased phosphorylation of ERK1/2. However, a more robust increase in ERK1/2 phosphorylation was observed for cells plated on the αC(FXIII) oligomers, particularly in the case of ERK1. Thus, adhesion-dependent phosphorylation of ERK1/2 is also enhanced by oligomerization of the αC domain.
Discussion
Interaction of fibrin(ogen) with endothelial cells occurs through a number of cell receptors. They include vascular endothelial (VE)–cadherin, intercellular adhesion molecule-1 (ICAM-1), and at least 2 integrins, αVβ3 and α5β1, which interact with complementary binding sites located in different fibrin(ogen) domains. In particular, the βN domains of the central E region interact with VE-cadherin,38 the γ chain sequences located in the coiled coil and γC domains of the D regions interact with ICAM-1 and αVβ3 integrin, respectively,39,40 and the αC domains interact with αVβ3 and α5β1.5,7,23,41 In this study, we have examined the mechanism of the αC domain–mediated interaction of fibrinogen and fibrin with endothelial cells. Notably, in fibrinogen the αC domains are monomeric/dimeric, while in fibrin they form ordered cross-linked polymers24 in which their RGD-containing and other binding sites are brought into close proximity. Because of this structural difference, we tested both the monomeric αC domains and the transglutaminase–cross-linked αC oligomers that mimic the arrangement and properties of these domains in fibrinogen and fibrin, respectively.28
Factor XIII (FXIII) and tissue transglutaminase (tTG) are the most abundant members of transglutaminase family. Proenzyme FXIII, for which fibrin is the major physiological substrate, is present in plasma and platelets, whereas tTG is localized in the extracellular matrix and on the surface of various cell types. tTG is particularly abundant on the basal surface of endothelial monolayer in blood vessels.42,43 Moreover, it is enzymatically active and is able to cross-link fibrinogen on the surface of endothelial cells in situ.44 Thus, when the endothelial lining is damaged and tTG becomes exposed, it may substantially contribute to the cross-linking of fibrin. Therefore, in this study we prepared αC domain oligomers cross-linked with tTG, αC(tTG), and compared them with FXIIIa–cross-linked αC domain oligomers, αC(FXIII). The experiments demonstrated that both transglutaminases produced αC oligomers with similar properties. Both types of oligomers, αC(FXIII) and αC(tTG), had similar ordered structure and exhibited dramatically increased adhesion capacity for HUVECs in comparison with that of the monomeric αC domains.
Previous work showed that 2 integrins on endothelial cells, αVβ3 and α5β1, mediate their interaction with fibrin(ogen) via the RGD sequence of the αC domains.5,7,23,41,45 Cell adhesion experiments with isolated αC domain monomers/oligomers and function-blocking monoclonal antibodies presented here confirmed those findings and revealed that another integrin, αVβ5, is also involved in RGD-dependent adhesion of endothelial cells to fibrin via its αC domains. The relative contribution of each integrin to the adhesion process was found to be different. While blocking anti-αVβ5 and anti-α5β1 mAbs inhibited adhesion by only 15% to 30%, the mAb against αVβ3 reduced adhesion by about 75%. A similar pattern was obtained by chemical cross-linking of endothelial integrins to the αC domain species. Altogether, these results indicate that HUVECs utilize all these 3 integrins for the interaction with the fibrin(ogen) αC domains, with αVβ3 playing a predominant role in this process. Because our estimates showed that HUVECs contain similar numbers of these integrin receptors, such a prominent role of αVβ3 could be explained by its potentially higher affinity for the αC domains compared with those of α5β1 and αVβ5 integrins.
Our findings clearly indicate that transglutaminase-mediated oligomerization of the αC domains not only substantially promotes adhesion of HUVECs but also leads to dramatic changes in the adherent cells, including increased spreading and formation of large integrin clusters within the peripheral focal adhesions. All these effects are most likely a result of the oligomeric structure of the αC domains. Because the density of ligands and their affinity for cell receptors are among the key factors influencing receptor clustering into focal adhesions,46-48 it is obvious that oligomerization of the αC domains, which increases local density by juxtaposition of their RGD-containing integrin-binding sites, should enhance integrin clustering on endothelial cells. Further, oligomerization of the αC domains may modulate their affinity for endothelial integrins. In agreement, our solid-phase binding experiments revealed an increase in the interaction of the isolated αVβ3 integrin with αC oligomers compared with that with αC monomers. The structural basis for such modulation of affinity is less obvious. One can only speculate that oligomerization of the αC domains induces conformational changes altering presentation of their integrin-binding sites or increases their affinity by some other mechanisms. In addition, transglutaminase-mediated covalent cross-linking of Lys residues located in the vicinity of Aα572-574 RGD (such as Lys556 and/or Lys580)29 may also affect the conformation of this site and increase integrin binding.
Our study also demonstrated that integrin clustering and formation of prominent focal adhesions by endothelial cells adherent to the αC oligomers amplifies integrin-mediated activation of FAK and ERK1/2 signaling pathways. FAK and ERK1/2 protein kinases serve as key intermediates in multiple signaling pathways triggered by engagement of various integrins, including αVβ3, α5β1, and αVβ5.49 Their sustained activation depends on integrin ligation and is required for survival, cell cycle progression, and cell migration. Several studies demonstrated the importance of FAK50,51 and ERK52,53 in the directional migration of endothelial cells and formation of new blood vessels. Therefore, enhanced activation of FAK and ERK in endothelial cells upon interaction with the αC oligomers may stimulate cell locomotion during angiogenesis.
The involvement of fibrin and αVβ3 integrin in angiogenesis is well established. It was shown that fibrin gels induce an angiogenic response54 and that an endothelial cell monolayer sandwiched between 2 fibrin gels rearranges into a network of capillary-like tubes.55 The suggested mechanism underlying fibrin-induced angiogenesis includes interaction of the NH2-terminal regions of the fibrin β chains with endothelial cell receptor VE-cadherin.38 Because the integrin αVβ3 is prominently involved in angiogenesis56 and αVβ3-mediated interaction of HUVECs with the αC domain oligomers results in increased adhesiveness, we anticipate an important role of this interaction in endothelial cell migration and formation of new blood vessels associated with wound healing and tumor progression. The relative contributions of integrin- and VE-cadherin–dependent mechanisms to fibrin-induced angiogenesis remain to be established.
It was proposed earlier that enzymatic activity of transglutaminases stabilizes basement membranes and interstitial matrices by increasing their resistance against mechanical and other stresses.43,57 In addition to fibrin(ogen), tTG and FXIIIa cross-link a number of other plasma and extracellular matrix proteins, including fibronectin, vitronectin, collagen, and osteopontin.43 Despite a wide occurrence of transglutaminase cross-links in a number of extracellular proteins, very little is known about their effects on functional activities of the affected proteins. It has been shown that transglutaminase-mediated cross-linking of fibrinogen to fibronectin stimulates adhesion to the covalent complexes of these proteins.58,59 Here we demonstrate a novel aspect of the involvement of transglutaminases in cell-matrix interactions. Namely, this study shows that transglutaminase-mediated formation of αC domain polymers in fibrin enhances their adhesive capacity for endothelial cells. Thus, transglutaminases may promote integrin-mediated cell-matrix interactions via enzymatic modification of integrin ligands.
In summary, this study revealed that transglutaminase-mediated oligomerization of the αC domains strongly stimulates RGD-dependent adhesion of endothelial cells via interactions with αVβ3 and to a lesser extent with αVβ5 and α5β1 integrins. These interactions result in increased integrin clustering, enhanced cell spreading, and amplification of integrin-dependent signaling, which is known to regulate endothelial cell migration and angiogenesis. The underlying mechanism for these effects is likely based on ordered juxtaposition of the RGD-containing integrin-binding sites upon oligomerization of the αC domains and their increased affinity for integrin receptors. The relationship between these cell-matrix interactions and endothelial cell migration during angiogenesis and wound healing remains to be explored.
Prepublished online as Blood First Edition Paper, January 6, 2005; DOI 10.1182/blood-2004-10-4089.
Supported by National Institutes of Health grants GM62895 (A.M.B.), HL30954 (J.W.W.), and HL-56051 (L.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal