Abstract
Severe sepsis leads to long-term systemic and local immunosuppression, which is the cause of a number of complications, including pulmonary infection. A therapeutic strategy that reverses this immunosuppression is required, given the ongoing high mortality rate of patients who have survived a severe sepsis. The present study demonstrates that experimental severe sepsis renders the lung susceptible to a normally innocuous Aspergillus fumigatus fungus challenge, due to a dominant lung type 2 cytokine profile. Dendritic cells (DCs) obtained from the lungs of mice subjected to cecal ligation and puncture (CLP) model were skewed toward type 2 cytokine profile, which occurred with exaggerated expression of Toll-like receptor 2 (TLR2). The intrapulmonary transfer of bone marrow–derived DCs (BMDCs) in postseptic mice prevented fatal Aspergillus infection. This therapy reduced the overall inflammatory response and fungal growth in the lung, and promoted the balance of proinflammatory and suppressive cytokines in the lung. Thus, intrapulmonary DC supplementation appears to restore the pulmonary host response in the postseptic lung in our animal model. These data strongly suggest that lung DCs are profoundly affected as a consequence of the systemic impact of severe sepsis, and the identification of mechanisms that restore their function may serve as a key strategy to reverse sepsis-induced immunosuppression.
Introduction
Host defense against pathogenic microorganisms requires the coordinated actions of the innate and acquired immune system. However, dysregulation of the immune system occurs during severe sepsis, leading to either a rapid death due to the development of multiorgan failure or an increase in complications due to long-term immunosuppression.1-6 Although the pathogenesis of the acute septic response has been extensively studied, the long-term consequence of a septic episode on the immune system is poorly characterized. Clinical studies have shown that postseptic patients are more susceptible to diseases such as pneumonia, cancer, and heart disease.7,8 In addition, the severity of septic episodes correlates with the time of death after discharge from hospital care.7,8
Aspergillus fumigatus is an innocuous fungus for the immunocompetent host, but it is a life-threatening pathogen for the immunocompromised host, such as a bone marrow transplant recipient and a patient with neutropenia due to disease or drug-related immunosuppression.1,9,10 The antifungal host response requires an intact type 1 cytokine response to enable macrophages11 and neutrophils12 to eliminate fungal spores and impair hyphae growth, respectively.1,9,10 More recently, it has been shown that dendritic cells (DCs) orchestrate the overall pulmonary antifungal immunity, including driving the T helper 1 (Th1)/Th2 type response.13 Specifically, Aspergillus-pulsed DCs conferred resistance to aspergillosis or candidiasis in mice subjected to allogeneic hematopoietic stem cell transplantation model.14,15 These data highlight the importance of the integration of the innate and acquired immune response during antifungal responses.
Recently, we described a model in which mice that survive severe sepsis rapidly succumbed to A fumigatus infection, whereas sham-operated mice were resistant to the same challenge.16 In the present study, mice subjected to cecal ligation and puncture (CLP) model remained susceptible to a fungal infection at days 5 and 15 after CLP surgery. We now report that this long-term susceptibility appears to be due to the skewing of the pulmonary cytokine pattern toward type 2 responses, due in part to altered cytokine generation by DCs. More strikingly, the intrapulmonary instillation of bone marrow–derived DCs (BMDCs) into the lungs of postseptic mice restored an effective antifungal host response in the lung.
Materials and methods
Reagents
Escherichia coli lipopolysaccharide (LPS) 055:B5, Pam3Cys-Ser-(Lys)4 (Pam3Cys), and Poly(I:C) were purchased from Sigma (St Louis, MO), from EMC microcollections (Tuebingen, Germany), and from Amersham Bioscience (Uppsala, Sweden), respectively. Shistosoma mansoni eggs antigen (SEA) was purified as described elsewhere.17 A fumigatus conidia was purchased from American Type Culture Collection (ATCC, Manassas, VA), and A fumigatus conidia antigen was obtained from Greer Laboratories (Lenoir, NC). Fluorescein isothiocyanate (FITC)–conjugated rat immunoglobulin G (IgG) directed to murine I-Ab, CD80, and CD40, and phycoerythrin (PE)–conjugated rat IgG directed to murine CD11c were from Pharmingen (Palo Alto, CA).
Mice
Specific pathogen-free, female, C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME) and maintained under specific pathogen-free conditions. The University of Michigan Committee on Use and Care of Animals approved all animal studies outlined in this article. Green fluorescent protein (GFP)–transgenic (Tg) mice on C57BL/6 background were kindly provided by Dr Steven Chensue (University of Michigan, Ann Arbor, MI), who obtained them from Dr Sergio Lira (Schering-Plough Research Institute, Kenilworth, NJ).18
CLP model
Animals were subjected to sham or CLP surgery as previously described.19 Mice in both surgery groups were treated with an antibiotic preparation containing imipenem conjugated with cilastatin (10 mg/kg intraperitoneally; Merck, West Point, PA) beginning at 7 hours after CLP surgery and readministered every 12 hours thereafter until day 3 after surgery. Antibiotic therapy16,20 increased mouse survival to the 60% to 70% range, and these mice were used in the following experiments. Sham-operated (n = 10) and CLP (n = 20) mice were weighed daily beginning prior to surgery and continuing for 15 days after surgery. Unless otherwise stated, sham and CLP mice were used for all experiments at day 15 after the surgery.
A fumigatus culture conditions and intrapulmonary infection
In order to examine alterations in the pulmonary innate immune response after sepsis, we examined the impact of intratracheally injected A fumigatus conidia in mice previously subjected to sham or CLP surgery and antibiotic therapy.16 At day 15 after sham or CLP surgery, each mouse was anesthetized and received an intratracheal injection of 5 × 107A fumigatus conidia suspended in 30 μL phosphate-buffered saline (PBS) as described elsewhere.21 Mouse survival and pulmonary response were monitored for up to 10 days after the A fumigatus conidia challenge.
Lung histology
Lungs were collected at day 2 after the intratracheal conidia challenge in sham or CLP surgery groups. Whole-lung samples for histologic examination were excised, perfused with 10% formalin, and placed in fresh formalin for an additional 24 hours. Routine histologic techniques were used to paraffin-embed this tissue, and 3-μm sections of whole lung were stained with hematoxylin and eosin (H&E) or with Gomori methanamine silver (GMS) to localize A fumigatus conidia and hyphal elements (black staining). Image capture was carried out by an Olympus BX40F microscope equipped with 20×/0.5 and 100×/1.3 objective lenses and a 10× eyepiece (Olympus, Melville, NY). Immersion oil was used for higher magnification. Digital photographs were obtained with a Sony 3CCD color video camera, model number DXC-960MD (Sony, Tokyo, Japan), and IP Lab Spectrum software was used for image acquisition (Scanalytics, Fairfax, VA).
Isolation of mouse BMDCs
DCs were generated from BM cells harvested from 8- to 10-week-old C57BL/6 or GFP-C57BL/6 female naive, sham-operated (nonseptic) or CLP surgery (septic) mice.22 BM cells were flushed out from the femurs and tibias. After lysis of red blood cells (RBCs), whole BM cells (1 × 106/mL) were cultured in complete medium containing 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). Supplemented media were replaced after 3 days. Following 7 days of culture, morphologically distinct BMDCs were harvested, resuspended at 5 to 10 × 106 cells/mL, and centrifuged through an iodixanol gradient (11.5%) (AXIS-SHIELD, Oslo, Norway). Purified BMDCs were collected from the gradient interface, resuspended, and injected at 1 × 106/30 μL in PBS/mouse. Unless otherwise stated, the BMDCs used for transfer were obtained from naive mice.
DC transfer
BMDCs were injected alone (1 × 106/30 μL) or coinjected with A fumigatus conidia (5 × 107/30 μL) intratracheally. Mice were killed at determined times and the lungs were used for flow cytometry, lung histology, immunohistochemistry, enzyme-linked immunosorbent assay (ELISA), and mRNA expression by Taqman. In one set of experiments, mice were subjected to CLP and challenged with conidia alone or coinjected with conidia and BMDCs/naive at day 5 after surgery. The survival was evaluated for 7 days. In another set of experiments, mice were subjected to CLP and at day 15 challenged with conidia alone or coinjected with conidia and BMDCs obtained from the 15-day sham group (BMDC/sham) or CLP group (BMDC/CLP). The lungs were collected 10 days after challenge and processed for GMS staining.
Clinical chemistry
Serum levels of aspartate transaminase (AST), alanine transaminase (ALT), creatinine, and bilirubin were measured by Clinical Pathology at the University of Michigan Medical School using standardized techniques.
Bronchoalveolar lavage (BAL)
At 6 hours or 2 days after saline or A fumigatus intratracheal injection, groups of at least 5 mice were killed by anesthesia overdose, the trachea was exposed, and a 1-mL syringe with 18-gauge needle was inserted into the trachea. BAL was performed by instilling and recovering 1 mL normal saline. BAL samples were cytospun onto microscope slides and cells were stained and counted using the Diff-Quick method (Baxter Travenol Laboratories, Mississauga, ON, Canada).
Measurement of myeloperoxidase (MPO)
Immunoreactive MPO levels were measured by ELISA in tissue extracts (Calbiochem-Novabiochem, San Diego, CA) according to the manufacturer's instructions.
Cytokine and chemokine ELISA analysis
At day 15 after sham or CLP surgery, mice were killed by anesthesia overdose. Lung lobes were homogenized in 1 mL lysis buffer PBS, 0.5% triton X-100, and protease inhibitor (Roche Diagnostics, Mannheim, Germany) using a tissue homogenizer. Tissue-free supernatants were analyzed for murine CC chemokine ligand 2 (CCL2) monocyte chemoattractant protein-1 (MCP-1), IL-13, transforming growth factor β (TGF-β), IL-12, IL-6, and IL-4 using a modified double-ligand ELISA assay as previously described.23 Whole-lung levels were also measured at day 2 after A fumigatus conidia challenge in mice subjected to sham or CLP surgery. The results were normalized to the amount of protein present in cell-free preparations of each sample by Bradford assay.24
Lung DC culture
Whole lungs from 7 to 10 sham and CLP mice were dispersed by collagenase digestion as described above, red blood cells (RBCs) were lysed, and total cells were plated. Nonadherent cells were collected, and up to 108 of these cells were resuspended in 400 μL buffer (1 × PBS/0.5% bovine serum albumin) containing 100 μL CD11c microbeads (MACS Miltenyi Biotec, Aubum, CA). This suspension was incubated at 4°C for 15 minutes. The free beads were washed away and the cells with beads were added to a MS+/RS+ column (MACS Miltenyi Biotec) for positive selection. The purified DCs were subsequently diluted at 1 × 106/mL, and 200 μL of this suspension was added to a 96-well plate. Plated DCs were stimulated with medium (complete RPMI), LPS (1 μg/mL), poly(I:C) (100 μg/mL), SEA (100 μg/mL), Pam3cys (25 μg/mL), A fumigatus conidia antigen (50 μg/mL), or A fumigatus conidia (1:1 ratio) for 48 hours. Supernatants from all treatment groups were collected for ELISA.
Flow cytometry
Blocking was performed by incubating cells with purified rat anti–mouse CD16/CD32 (FcγIII/II receptor) monoclonal antibody from Pharmingen. The data were obtained from gated in side scatter (SSC)/forward scatter (FSC) dendritic cell. The lungs were collected after sham or CLP surgery, or after secondary conidia challenge or saline injection. The flow cytometry analysis was performed with a FACSCalibur (CELLQuest software; Becton and Dickinson, Mountain View, CA). Cells were gated on CD11C+ cells, and the percentages of the second marker were calculated.
Tracking of DCs
At indicated times (1, 3, 7, and 15 days after sham or CLP surgery, or 1 day after A fumigatus conidia challenge), mice that received an intratracheal injection of DCs with or without A fumigatus conidia were killed, and mesenteric and lung lymph nodes, spleen, and BAL were collected and manually passed through a cell strainer. The lung samples were minced and cells were then dispersed by treatment with 0.2% of collagenase type IV (Sigma) at 37° for 45 minutes. All cell suspensions were incubated with antibody, and flow cytometry was used as described in “Flow cytometry.”
In another set of experiments, mice received an intratracheal injection of GFP-DCs 1 day before sham or CLP surgery. One day later, mice were killed and the same tissues were collected and processed as described in this section.
Real-time TaqMan PCR analysis
Individual mouse lungs or plated DCs were harvested and homogenized, and the total RNA was isolated using the Trizol reagent (Invitrogen/Life Technologies, Carlsbad, CA). Once isolated, 5 μg total RNA from lung tissue or 10 μL total material from plated DCs was reverse transcribed to yield cDNA, and Toll-like receptor 2 (TLR2) gene expression was analyzed by real-time quantitative reverse-transcriptase–polymerase chain reaction (RT-PCR) procedure using an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). Glyceraldehyde phosphate dehydrogenase (GAPDH) was analyzed as an internal control. The TLR2 fluorogenic PCR system consisted of the amplification primers and probe: forward primer, 5′-GCC ACC ATT TCC ACG GAC T-3′; reverse, 5′GGC TTC CTC TTG GCC TGG-3′; probe, 5′ G-carboxyl-fluorescein (FAM)–TGG TAC CTG AGA ATG ATG TGG GCG TG-3′ 6-carboxy-tetramethylrhodamine (TAMRA).25 The primers and probe were purchased from Applied Biosystems. Gene expression was normalized to GAPDH before the fold change in gene expression was calculated. The mRNA level of naive mouse or from sham DCs treated with medium was assigned an arbitrary value of 1.
Statistical analysis
All data shown are means ± SE and are representative of 2 to 4 separate experiments. The means between different treatments were compared by analysis of variance. When significance was detected, individual differences were analyzed using the Bonferroni t test for unpaired values. Statistical significance was set at P less than .05. Survival rates were expressed as percentages, and a log-rank test (χ2 test) was used to detect differences in mouse survival.
Results
Long-term pulmonary immunosuppression follows CLP surgery despite initial clinical recovery
We described that mice subjected to CLP, but not sham (ie, nonseptic), surgery are acutely susceptible to a secondary fungal infection, which persists for at least 15 days after surgery. Sham and CLP mice were intratracheally challenged with 5 × 107A. fumigatus conidia at day 15 after surgery, and mouse survival was monitored for 7 days. All mice in the CLP group were dead at 4 days after A fumigatus conidia challenge, while all the sham-operated mice challenged with same number of conidia were alive (Figure 1A). In CLP mice, a dramatically more intense inflammatory response was present around the airways (Figure 1Bii), compared with sham-operated mice (Figure 1Bi). GMS staining for fungal material revealed intact, live conidia and hypha growth in CLP mice but not in the sham group. (Figure 1Biii-vi). These data suggested that postseptic mice failed to eliminate A fumigatus from the lung despite the presence of inflammation.
Survival curve, lung histopathology, and neutrophil migration of sham- or CLP-operated mice challenged or not with A fumigatus. (A) At day 15 after sham or CLP surgery, both groups of mice were challenged intratracheally with either saline or 5 × 107A fumigatus conidia, and mouse survival was analyzed up to 7 days after challenges. Each group contained 8 to 10 mice, and data are representative of 2 independent experiments. *P < .05 between sham + Asp and CLP + Asp. (B) Pulmonary inflammatory change after A fumigatus conidia challenge in sham (i,iii,v) and CLP (ii,iv,vi) groups. At day 15 after surgery, all mice were challenged via intratracheal injection with saline or 5 × 107 conidia and were killed at day 2. The lung was collected and processed for histology. Paraffin-embedded lung sections were stained with H&E (i-ii) or GMS (iii-vi; fungal material appears black in lung sections [black dots]). Red arrows highlight dead conidia, while blue arrows highlight hyphae growth. Original magnification was × 20 for panels i-iv, and original magnification was × 100 for panels v-vi. (C) Sham and CLP surgery groups were intratracheally challenged with A fumigatus conidia at day 15 after surgery, and BAL was collected at 6 hours and 2 days after fungal challenge. The results shown are expressed as the percentage of neutrophils in the BAL, and each group contained 5 to 6 mice. The data are representative of 3 separate experiments, and are expressed as mean ± SEM. (D) Lung samples were obtained from sham- or CLP-operated mice challenged with either saline or 5 × 107A fumigatus conidia at day 2 after challenge. The tissue was processed for MPO protein concentration assay. The data are expressed as mean ± SEM; each group contained 10 to 12 mice.
Survival curve, lung histopathology, and neutrophil migration of sham- or CLP-operated mice challenged or not with A fumigatus. (A) At day 15 after sham or CLP surgery, both groups of mice were challenged intratracheally with either saline or 5 × 107A fumigatus conidia, and mouse survival was analyzed up to 7 days after challenges. Each group contained 8 to 10 mice, and data are representative of 2 independent experiments. *P < .05 between sham + Asp and CLP + Asp. (B) Pulmonary inflammatory change after A fumigatus conidia challenge in sham (i,iii,v) and CLP (ii,iv,vi) groups. At day 15 after surgery, all mice were challenged via intratracheal injection with saline or 5 × 107 conidia and were killed at day 2. The lung was collected and processed for histology. Paraffin-embedded lung sections were stained with H&E (i-ii) or GMS (iii-vi; fungal material appears black in lung sections [black dots]). Red arrows highlight dead conidia, while blue arrows highlight hyphae growth. Original magnification was × 20 for panels i-iv, and original magnification was × 100 for panels v-vi. (C) Sham and CLP surgery groups were intratracheally challenged with A fumigatus conidia at day 15 after surgery, and BAL was collected at 6 hours and 2 days after fungal challenge. The results shown are expressed as the percentage of neutrophils in the BAL, and each group contained 5 to 6 mice. The data are representative of 3 separate experiments, and are expressed as mean ± SEM. (D) Lung samples were obtained from sham- or CLP-operated mice challenged with either saline or 5 × 107A fumigatus conidia at day 2 after challenge. The tissue was processed for MPO protein concentration assay. The data are expressed as mean ± SEM; each group contained 10 to 12 mice.
Metabolic parameters were next evaluated in order to identify possible explanations for the immunosuppression observed after severe sepsis. In the present study, the sham group showed a minor decline in body weight (reduced by ∼ 6.89%) at day 1 after surgery, but these mice had recovered the lost body weight by day 5 (data not shown). In contrast, the CLP surgery group exhibited more dramatic initial weight loss (reduced by ∼ 13.15%); however, at day 15 after surgery both groups of mice exhibited similar body weight. Systemic inflammatory markers were also examined at day 15 after surgery, and serum levels of AST, ALT, creatinine, and bilirubin in CLP mice were similar to levels measured in sham-operated mice (data not shown). Also, the histologic appearance of the lung was unremarkable in the 2 surgery groups (data not shown). At this time, the sham and CLP mice were indistinguishable based on a number of clinical parameters including body mass, liver and kidney function, and lung histology.
Although the lungs from A fumigatus–challenged CLP mice showed evidence of overt inflammation, we assessed whether impaired leukocyte migration into the airspaces could have accounted for the development of invasive aspergillosis in these mice. At 6 hours and 2 days after conidia challenge, both groups exhibited BAL that contained approximately 60% to 80% neutrophils (Figure 1C). The percentage of macrophages in the BAL did not differ between the sham and CLP surgery groups at these times after A fumigatus challenge (data not shown). Finally, MPO protein levels were similar between surgery groups (Figure 1D). The susceptibility of CLP mice could not be explained by the absence of neutrophils and macrophages in the lung.
Type-2 cytokine levels are increased in the postseptic lung
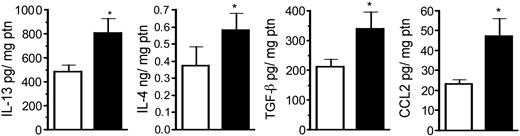
In order to determine the lung cytokine environment after CLP surgery, whole-lung cytokine and chemokine levels in sham and CLP groups at day 15 after surgery are shown in Figure 2. Previously, we observed that whole lung IL-13, IL-4, C10, and CCL2 (MCP-1) levels were significantly elevated at day 7 after CLP compared with sham-operated mice.16 In the present study, we observed that whole lung IL-13, IL-4, TGF-β, and CCL2 remained significantly elevated at day 15 compared with the sham group (Figure 2). No differences were observed in whole-lung IL-10 and tumor necrosis factor α (TNF-α) levels, while IL-12 levels were lower (P < .06) in the CLP group.
Whole-lung IL-13, IL-4, TGF-β, and CCL2 levels in sham- and CLP-operated mice at day 15 after surgery. Whole-lung samples were collected at day 15 and homogenized, and cell-free supernatant was analyzed by specific ELISA. The data (expressed as mean ± SEM) shown are representative of 2 separate experiments, and each group contained 6 to 7 mice. *P < .05 between sham (□) and CLP (▪) groups.
Whole-lung IL-13, IL-4, TGF-β, and CCL2 levels in sham- and CLP-operated mice at day 15 after surgery. Whole-lung samples were collected at day 15 and homogenized, and cell-free supernatant was analyzed by specific ELISA. The data (expressed as mean ± SEM) shown are representative of 2 separate experiments, and each group contained 6 to 7 mice. *P < .05 between sham (□) and CLP (▪) groups.
BMDCs from nonseptic, but not postseptic, mice protected CLP mice from A fumigatus infection
Considering that DCs are the major initiators of a specific immune response to pathogens, we hypothesized that an alteration in DC function may have accounted for the immunosuppression following sepsis. To address this question we transferred BMDCs obtained from naive mice (nonseptic BMDCs; “Materials and methods”) into CLP mice (at 5 days after CLP), concomitantly with an intratracheal injection of A fumigatus conidia. Figure 3A illustrates that the intrapulmonary transfer of BMDCs protected mice from mortality. Lung samples obtained from mice that received BMDCs/sham and conidia showed markedly fewer conidia and pulmonary inflammation 10 days after conidia challenge compared with mice challenged with conidia alone (Figure 3B). Consequently, mice that received BMDCs grown from 15-day CLP mice exhibited no improvement in fungal clearance (Figure 3Biii). The inflammatory response in these mice mirrored that observed in CLP mice that received conidia alone (Figure 3B). Thus, 2 important observations followed these experiments: (1) intrapulmonary DCs from non-CLP mice protected postseptic mice from fatal fungal growth and (2) intrapulmonary DCs were profoundly altered by the systemic effects of the septic event.
Survival curve and lung histopathology from CLP-operated mice challenged with A fumigatus alone or coinjected with BMDCs. (A) At day 5 after CLP surgery, groups of mice were challenged intratracheally with either 5 × 107 conidia alone or coinjected with BMDCs, and mouse survival was analyzed up to 7 days after A fumigatus challenge. Each group contained 5 mice. P < .05 between CLP + Asp and CLP + Asp + BDMC. (B) At day 15 after surgery, all mice were challenged via intratracheal injection with 5 × 107 conidia alone (i) or together with 1 × 106 BMDCs obtained from 15-day sham-operated mice (ii) or obtained from 15-day CLP mice (iii). The lungs were collected at day 10 after challenge and processed for histology. Paraffin-embedded lung sections were stained with GMS (fungal material appears black in lung sections [black dots]). Original magnification was × 20 for all panels.
Survival curve and lung histopathology from CLP-operated mice challenged with A fumigatus alone or coinjected with BMDCs. (A) At day 5 after CLP surgery, groups of mice were challenged intratracheally with either 5 × 107 conidia alone or coinjected with BMDCs, and mouse survival was analyzed up to 7 days after A fumigatus challenge. Each group contained 5 mice. P < .05 between CLP + Asp and CLP + Asp + BDMC. (B) At day 15 after surgery, all mice were challenged via intratracheal injection with 5 × 107 conidia alone (i) or together with 1 × 106 BMDCs obtained from 15-day sham-operated mice (ii) or obtained from 15-day CLP mice (iii). The lungs were collected at day 10 after challenge and processed for histology. Paraffin-embedded lung sections were stained with GMS (fungal material appears black in lung sections [black dots]). Original magnification was × 20 for all panels.
DCs harvested from postseptic lung were skewed to type-2 cytokine production
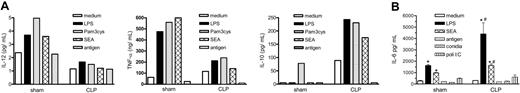
We next evaluated the cytokine profile of DCs from sham and CLP mice. Isolated lung DCs from both groups were stimulated with different agonists for 48 hours, prior to supernatant removal for cytokine analysis. DCs from CLP mice released markedly greater IL-10 and less IL-12 and TNF-α when stimulated with LPS (a TLR4 agonist26 ), Pam3cys (a TLR2 agonist27 ), or SEA (TLR2 agonist28 ) compared with similarly activated DCs obtained from sham mice (Figure 4A). Aspergillus conidia antigen did not induce detectable cytokine release. We also evaluated IL-6 levels in the supernatants due to the fact that IL-6 is an inflammatory cytokine that has been implicated in type 2 immune events.29 Cultured DCs purified from postseptic mice and activated with LPS or SEA released higher levels of IL-6 compared with DCs collected from sham mice, supporting the shift to a type 2 phenotype (Figure 4B). DCs were also exposed to poly(I:C), A fumigatus conidia alone, or the antigen of the A fumigatus conidia, but IL-6 release did not differ between the groups.
Soluble levels of IL-12, TNF-α, IL-10, and IL-6 from purified lung DCs obtained on day 15 after sham or CLP surgery. (A) Mice were subjected to sham or CLP surgeries and killed on day 15, and whole lung samples were processed. Lung DCs were purified by positive selection with CD11c beads. Purified DCs were plated at a density of 2 × 105/200 μL/well overnight, and then stimulated with medium, LPS (1 μg/mL), Pam3cys (25 μg/mL), SEA (100 μg/mL), or Aspergillus antigen (50 μg/mL) for 48 hours. Cell-free supernatants were collected, and IL-12, TNF-α, and IL-10 levels were measured by ELISA. (B) DCs were isolated and purified as described in panel A, and then cultured lung DCs were stimulated with medium, LPS (1 μg/mL), the legend regarding SEA (100 μg/mL), Aspergillus antigen (50 μg/mL), and Aspergillus conidia (1:1 ratio) and poli(I:C) (100 μg/mL) for 48 hours. Cell-free supernatants were collected and IL-6 level was measured by ELISA. Data are expressed as mean ± SEM. *P < .05 compared with the medium-treated group; #P < .05 compared with cytokine levels detected in cell-free supernatants from sham surgery lung DCs.
Soluble levels of IL-12, TNF-α, IL-10, and IL-6 from purified lung DCs obtained on day 15 after sham or CLP surgery. (A) Mice were subjected to sham or CLP surgeries and killed on day 15, and whole lung samples were processed. Lung DCs were purified by positive selection with CD11c beads. Purified DCs were plated at a density of 2 × 105/200 μL/well overnight, and then stimulated with medium, LPS (1 μg/mL), Pam3cys (25 μg/mL), SEA (100 μg/mL), or Aspergillus antigen (50 μg/mL) for 48 hours. Cell-free supernatants were collected, and IL-12, TNF-α, and IL-10 levels were measured by ELISA. (B) DCs were isolated and purified as described in panel A, and then cultured lung DCs were stimulated with medium, LPS (1 μg/mL), the legend regarding SEA (100 μg/mL), Aspergillus antigen (50 μg/mL), and Aspergillus conidia (1:1 ratio) and poli(I:C) (100 μg/mL) for 48 hours. Cell-free supernatants were collected and IL-6 level was measured by ELISA. Data are expressed as mean ± SEM. *P < .05 compared with the medium-treated group; #P < .05 compared with cytokine levels detected in cell-free supernatants from sham surgery lung DCs.
DC activation/trafficking in the postseptic lung
It has been shown that the expression of CD80, CD40, and major histocompatibility complex class II (MHC II) on DCs is critically important for their interaction or/and activation of T cells.30,31 DCs are necessary for effective antifungal responses.14 CLP mice showed a marked loss of CD11c+ cells in the lung at days 1, 3, and 7 after CLP. However, CD11c+ DC percentage was increased between the 2 groups at day 15 (Table 1). CD80 and CD40 expression did not differ between the groups at most times after CLP, but CD80 was increased in the CLP group at day 7. Pulmonary MHC II+ cells were significantly less abundant at day 3, and remained lower (not statistically significant) in the CLP group versus the sham group at all times examined after the induction of sepsis. It should also be noted that double-stained CD11c+ MHC II+ cells were less abundant in the CLP group at days 3, 7, and 15. Together, these data suggested that the persistent lack of immunocompetent cells in the lungs of CLP was not the likely explanation for the defective antifungal response in CLP mice at day 15 after sepsis.
Alterations in CD11c+ and MHC II+ DC populations in the lung after sham or CLP surgery
Marker/group . | 1 d, % gated . | 3 d, % gated . | 7 d, % gated . | 15 d, % gated . |
|---|---|---|---|---|
| CD11c | ||||
| Sham | 45.8 ± 2.8 | 30.11 ± 4.32 | 40.6 ± 2.18 | 19.83 ± 1.5 |
| CLP | 32.3 ± 1.4* | 19.6 ± 3.07 | 21.0 ± 3.1* | 28.8 ± 2.1* |
| MHC II | ||||
| Sham | 8.9 ± 2.7 | 5.3 ± 1.13 | 3.9 ± 0.59 | 3.9 ± 1.4 |
| CLP | 15.5 ± 4.8 | 0.7 ± 0.02* | 2.9 ± 1.54 | 1.62 ± 0.2 |
| CD80 | ||||
| Sham | 37.8 ± 5.3 | 21.7 ± 3.6 | 9.3 ± 1.13 | 20.9 ± 8.3 |
| CLP | 35.7 ± 6.7 | 12.8 ± 2.2 | 16.1 ± 1.69* | 17.8 ± 1.3 |
| CD40 | ||||
| Sham | 59.2 ± 5.4 | 42.8 ± 6.7 | 15.2 ± 0.44 | 29.3 ± 3.4 |
| CLP | 46.4 ± 7.1 | 32.7 ± 2.0 | 16.3 ± 1.89 | 32.2 ± 3.5 |
Marker/group . | 1 d, % gated . | 3 d, % gated . | 7 d, % gated . | 15 d, % gated . |
|---|---|---|---|---|
| CD11c | ||||
| Sham | 45.8 ± 2.8 | 30.11 ± 4.32 | 40.6 ± 2.18 | 19.83 ± 1.5 |
| CLP | 32.3 ± 1.4* | 19.6 ± 3.07 | 21.0 ± 3.1* | 28.8 ± 2.1* |
| MHC II | ||||
| Sham | 8.9 ± 2.7 | 5.3 ± 1.13 | 3.9 ± 0.59 | 3.9 ± 1.4 |
| CLP | 15.5 ± 4.8 | 0.7 ± 0.02* | 2.9 ± 1.54 | 1.62 ± 0.2 |
| CD80 | ||||
| Sham | 37.8 ± 5.3 | 21.7 ± 3.6 | 9.3 ± 1.13 | 20.9 ± 8.3 |
| CLP | 35.7 ± 6.7 | 12.8 ± 2.2 | 16.1 ± 1.69* | 17.8 ± 1.3 |
| CD40 | ||||
| Sham | 59.2 ± 5.4 | 42.8 ± 6.7 | 15.2 ± 0.44 | 29.3 ± 3.4 |
| CLP | 46.4 ± 7.1 | 32.7 ± 2.0 | 16.3 ± 1.89 | 32.2 ± 3.5 |
Mice were killed at days 1, 3, 7, and 15 after sham or CLP surgery. Whole-lung samples were collected and processed for fluorescence-activated cell sorting (FACS) analysis. Each value is a mean ± SEM of 3 to 4 mice, and the data are representative of 3 separate experiments.
P < .05 between sham and CLP groups for each time point
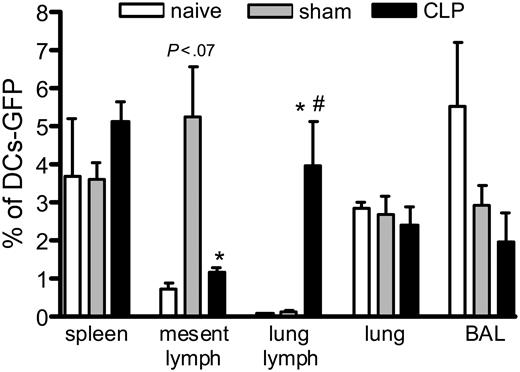
We also evaluated the migration of GFP-BMDCs when injected one day before sham or CLP operation, and we tracked the DCs one day after the surgery. Figure 5 shows that the DCs migrate mainly to spleen and lung lymph nodes in mice subjected to CLP, while DCs mainly migrate to the mesenteric lymph nodes and the spleen in the sham controls. The GFP-BMDCs were most often found in the spleen and BAL when injected in naive mice. Interestingly, the GFP-BMDCs administered to the CLP mice traffic preferentially to the lung lymph node over the mesenteric lymph nodes, even though the mesenteric area was undergoing a profound inflammatory response.
Different migration of GFP-BMDCs in mice subjected to CLP surgery. Mice received an intrathecal injection of 1 × 106 GFP-DCs, and 24 hours later they were subjected to sham or CLP surgery. One day after, they were killed and the spleen, mesenteric and lung lymph nodes, lung, and BAL were collected and processed for flow cytometry analysis. Each value is a mean ± SEM of 3 to 4 mice, and the data are representative of 2 experiments. *P < .05 between sham and CLP groups; and #P < .05 between naive and CLP.
Different migration of GFP-BMDCs in mice subjected to CLP surgery. Mice received an intrathecal injection of 1 × 106 GFP-DCs, and 24 hours later they were subjected to sham or CLP surgery. One day after, they were killed and the spleen, mesenteric and lung lymph nodes, lung, and BAL were collected and processed for flow cytometry analysis. Each value is a mean ± SEM of 3 to 4 mice, and the data are representative of 2 experiments. *P < .05 between sham and CLP groups; and #P < .05 between naive and CLP.
We next determined the presence of DCs, through CD11c+ expression, at day one after A fumigatus conidia challenge in the sham and CLP groups. At day 15, both groups received an intratracheal coinjection of 1 × 106 BMDCs and conidia. We observed that 70% of the cells in the BAL were CD11c+ in both sham and CLP groups that received intratracheal saline, but after A fumigatus conidia challenge fewer than 2% of the cells in this compartment were CD11c+ in both groups of mice (Table 2). In the lung, the A fumigatus conidia challenge significantly decreased the percentage of CD11c+ cells in both surgery groups, but the reduction (approximately 78%) was more dramatic in the sham plus conidia group compared with the CLP plus conidia group (approximately 56%). The presence of this cell type in the mesenteric and lung lymph nodes was not altered by CLP surgery or after conidia challenge. Interestingly, more CD11c+ cells were present in the spleen of CLP group challenged with conidia (Table 2). Thus, the distribution of DCs does not seem to differ markedly in the surgery groups.
Differential migration of BMDC/CD11c+ in mice subjected to CLP surgery and challenged with A fumigatus conidia at day 15
CD11c marker and group . | Sham + sal, % gated . | Sham + asp, % gated . | CLP + sal, % gated . | CLP + asp, % gated . |
|---|---|---|---|---|
| BAL | 69 ± 4.9 | 1.9 ± 0.32* | 72.6 ± 1.8 | 0.94 ± 0.5* |
| Lung | 20.0 ± 1.3 | 5.05 ± 0.16* | 10.1 ± 0.8† | 4.4 ± 0.37* |
| LLN | 20.2 ± 1.4 | 22.5 ± 0.75 | 20.3 ± 0.8 | 19.3 ± 3.6 |
| MLN | 11.2 ± 2.3 | 9.4 ± 1.0 | 6.5 ± 1.2 | 7.61 ± 1.3 |
| Spleen | 7.2 ± 1.2 | 8.7 ± 0.45 | 9.9 ± 1.5 | 18.4 ± 2.5*† |
CD11c marker and group . | Sham + sal, % gated . | Sham + asp, % gated . | CLP + sal, % gated . | CLP + asp, % gated . |
|---|---|---|---|---|
| BAL | 69 ± 4.9 | 1.9 ± 0.32* | 72.6 ± 1.8 | 0.94 ± 0.5* |
| Lung | 20.0 ± 1.3 | 5.05 ± 0.16* | 10.1 ± 0.8† | 4.4 ± 0.37* |
| LLN | 20.2 ± 1.4 | 22.5 ± 0.75 | 20.3 ± 0.8 | 19.3 ± 3.6 |
| MLN | 11.2 ± 2.3 | 9.4 ± 1.0 | 6.5 ± 1.2 | 7.61 ± 1.3 |
| Spleen | 7.2 ± 1.2 | 8.7 ± 0.45 | 9.9 ± 1.5 | 18.4 ± 2.5*† |
Mice were subjected to sham or CLP operation and at day 15 they received an intratracheal coinjection of 1 × 106 BMDCs plus 5 × 107 conidia, and 24 hours after they were killed and the tissues (spleen, mesenteric and lung lymph nodes, lung and BAL) were harvested for FACS analysis. Each value is a mean ± SEM of 3 to 4 mice, and the data are representative of 2 experiments.
sal indicates saline; asp, A fumigatus conidia; LLN, lung lymph node; and MLN, mesenteric lymph node.
P < .05 between sham with saline or conidia groups; or CLP with saline or conidia groups
P < .05 between sham and CLP with saline or conidia
The transfer of BMDCs into the lung restored a balance in the host response against fungal infection
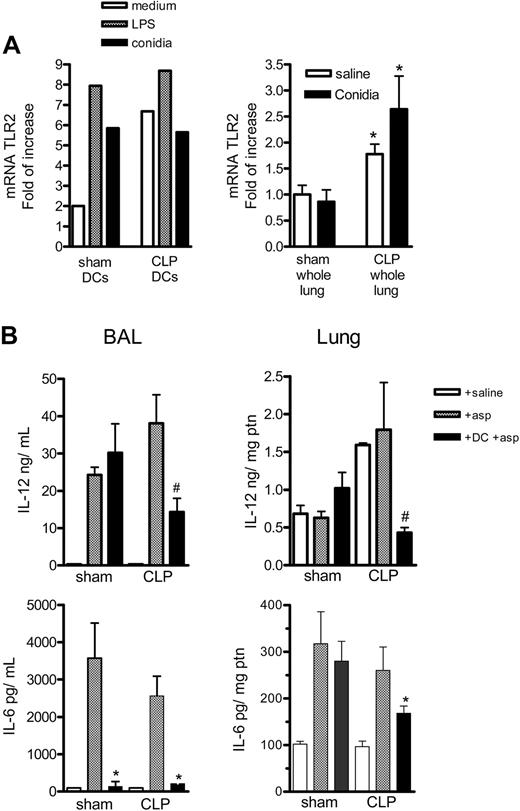
It has been previously shown that TLR2 expression correlates with a type 2 response,13 and A fumigatus conidia binds TLR2, which is both constitutively present and induced on DCs.31,32 We examined this TLR in sham and CLP mice infected with A fumigatus conidia. Supporting our suggestion that overwhelming TLR2 expression may be contributing to the shift to the type 2 response observed after sepsis, we observed that DCs purified from the lung CLP group, compared with the sham group, expressed more TLR2 mRNA at 4 hours of incubation with medium; but there was no increase after stimulation with LPS or conidia (Figure 6A). We also observed greater TLR2 mRNA expression in the lung of both saline and conidia treatment CLP mice at 6 hours, compared with sham controls.
TLR2 expression on purified DCs or whole lungs from sham-operated and CLP mice and IL-12 and IL-6 levels on whole lung of sham-operated and CLP mice that received DC therapy during fungal challenge. (A) Left graph shows the mRNA for TLR2 on DCs purified from sham or CLP mice 15 days after surgery. The DCs were plated at 2 × 105/200 μL, and, after resting, the cells were incubated with medium, LPS (1 μg/mL), or conidia (1:1) for 4 hours; cells were then harvested and the mRNA levels were evaluated by real-time PCR (Taqman). The right graph shows the mRNA TLR2 expression in whole lung of sham and CLP mice 15 days after surgery and 6 hours after challenge with saline or 5 × 107 conidia. *P < .05 between sham and CLP groups. The data shown are representative of 2 experiments. (B) Groups of mice were subjected to sham or CLP surgery, and at day 15 they received an intratracheal injection of saline, 5 × 107 conidia alone, or the same number of conidia plus 1 × 106 BMDCs. At day 2 after the intratracheal challenge, mice were killed, and BAL and lung tissues were processed for ELISA analysis. The data shown are representative of 2 experiments. *P < .05 compared with CLP + Asp group, and #P < .05 compared with Sham + DC + asp and CLP + asp. Data are expressed as mean ± SEM.
TLR2 expression on purified DCs or whole lungs from sham-operated and CLP mice and IL-12 and IL-6 levels on whole lung of sham-operated and CLP mice that received DC therapy during fungal challenge. (A) Left graph shows the mRNA for TLR2 on DCs purified from sham or CLP mice 15 days after surgery. The DCs were plated at 2 × 105/200 μL, and, after resting, the cells were incubated with medium, LPS (1 μg/mL), or conidia (1:1) for 4 hours; cells were then harvested and the mRNA levels were evaluated by real-time PCR (Taqman). The right graph shows the mRNA TLR2 expression in whole lung of sham and CLP mice 15 days after surgery and 6 hours after challenge with saline or 5 × 107 conidia. *P < .05 between sham and CLP groups. The data shown are representative of 2 experiments. (B) Groups of mice were subjected to sham or CLP surgery, and at day 15 they received an intratracheal injection of saline, 5 × 107 conidia alone, or the same number of conidia plus 1 × 106 BMDCs. At day 2 after the intratracheal challenge, mice were killed, and BAL and lung tissues were processed for ELISA analysis. The data shown are representative of 2 experiments. *P < .05 compared with CLP + Asp group, and #P < .05 compared with Sham + DC + asp and CLP + asp. Data are expressed as mean ± SEM.
Although BAL and lung levels of IL-6 and IL-12 from sham and CLP mice receiving saline alone were not different, A fumigatus conidia alone or A fumigatus conidia with BMDCs demonstrated significant differences. In the BAL, the IL-6 levels were lower in the sham and CLP group with conidia that received BMDC therapy compared with the group with conidia alone, while the IL-12 levels were lower in the BMDC plus conidia compared with the group with conidia alone, only in CLP mice. The lungs of CLP mice challenged with conidia plus BMDC exhibited statistically significant lower lung IL-6 and IL-12 levels, compared with the CLP group that received conidia alone (Figure 6B).
Discussion
Immunosuppression following sepsis remains a poorly understood clinical problem. Patients who survive severe sepsis appear to be at higher risk for ongoing clinical problems including infection, cancer, and other complications for up to 8 years after the septic episode.7,8 To explore this clinical dilemma, we recently developed a model in which mice surviving a severe septic episode were all susceptible to a pulmonary challenge with A fumigatus conidia, compared with sham-operated mice, which were completely resistant to A fumigatus challenge.16 Given the observation that only immunocompromised hosts are susceptible to this fungus, this model has provided an in vivo tool to explore sepsis-induced changes in host defense response. Data from the present study suggest that severe sepsis disrupts normal cytokine balance, and the immune response in the lung is skewed toward immunomodulation.9,33-36 We hypothesize that the failure of the postseptic host response may be a consequence of a shift in lung DC activation. This is supported by our data demonstrating that BMDC supplementation protected postseptic mice from fatal fungal infection and modulated the inflammatory response in the lung. Finally, BMDCs from postseptic mice did not reverse the immunosuppression, suggesting that sepsis impacted BMDCs and possibly explaining the observed long-term susceptibility of postseptic mice to Aspergillus. Interestingly, the immunosuppression in postseptic mice persisted despite the fact that the levels of many of the DC markers did not significantly differ from those of nonseptic mice, at day 15 after surgery.
The Shankar group demonstrated that bone marrow cells from burn injury and superimposed wound sepsis mice present increased bone marrow monocytopoiesis and peripheral monocytosis (Davis et al37 ). Also, the Miller-Graziano group (De et al38 ) showed that enhanced monocyte-to-macrophage differentiation with concomitant inhibited immature DC (iDC) development, partially due to increased circulating monocyte sensitivity to and production of macrophage colony-stimulating factor (M-CSF), occurred in trauma patients. They suggest that this contributes to the immunosuppression observed after injury.38
In regard to the DCs used for therapy, BMDCs from naive mice obtained after 7 days of incubation with GM-CSF/IL-4 and gradient purification contained less than 5% neutrophils, and approximately 90% of the cells presented dendrites, the characteristic morphology of DCs. In addition, approximately 60% of these cells were CD11c+, which expressed CD80 (66%), CD86 (48.2%), CD11b (78.3%), and MHC II (55.2%). Few cells were B220+ (23%) and few were CD8a+ (0.96%). These cells represent a myeloid DC population of intermediate maturation status, that is, not fully mature but also not immature. The surprising observation was that only a small increase in surface marker expression was observed between normal and CLP BMDCs after 7 days of incubation with GM-CSF/IL-4 (MHC II = 66.25%; CD80 = 83.05%; CD86 = 59.3%; CD11b = 73.1%; B220 = 30.35%; CD8α= 2.2%).
While DCs are clearly important in immune activation, little is known about their role in sepsis-induced immunosuppression.30 DCs exist in an immature form, which is a highly proficient cell in acquiring and processing antigen.39 Mature DCs are less able to process antigen but secrete large quantities of immune stimulatory cytokines that directly influence the type of immune response generated.31 In addition, DCs exhibit a remarkable functional plasticity in their discrimination between maturation stages of fungi (ie, conidia vs hyphae). This discrimination is highlighted by differential cytokine production profiles and the induction of Th cell reactivity in vitro and in vivo.9 We demonstrated that the presence of DCs in the lung was reduced from day 1 until day 7, but this CD11c+ population was restored and even more abundant at day 15 after CLP compared with the sham group. Strikingly, the I-Ab+ (MHC II+) population was increased at day 1 after CLP, but this population was significantly decreased at day 3 and remained in reduced numbers at all other times, compared with the sham group. Given that the sham group was resistant to the A fumigatus conidia challenge, while the CLP group was highly susceptible (100% mortality), the lack of I-Ab–positive DCs in the CLP group may have accounted for their susceptibility to Aspergillus. I-Ab expression by DCs is critical for antigen presentation to T cells, and the loss of this molecule impairs a key cell-to-cell interaction between these 2 cell types. However, the presence of DCs expressing CD80 and CD40 molecules, which are costimulatory molecule and activation markers, respectively, did not differ between the surgery groups. Thus, the absence of CD11c/I-Ab–positive cells in the lungs of CLP mice may have only partly accounted for the immune defect observed in these mice. In addition to these data, CD11c+ DCs were found mainly in the spleen of CLP mice at one day after Aspergillus challenge, which was not observed for the sham group. This alternative tracking of DCs after sepsis and secondary fungal infection suggests that alterations in chemokine release and/or chemokine receptor expression are present, and this alteration may influence the antifungal response.
Other research groups40-42 have demonstrated that there is a loss of DCs in the local and distant lymph nodes, particularly CD8+ lymphoid-derived DCs. These investigations suggest that this contributes to the alteration in the acquired immune status of septic mice.40 Also, these authors demonstrated a loss of interdigitating dendritic cells and follicular dendritic cells in both humans and mice, due to profound apoptosis, which significantly compromises B- and T-cell function. These data corroborate with ours, in that we observed a loss of DCs in the lung after sepsis, and the repopulated lung DCs promoted a type 2 response, contributing to the susceptibility of postseptic mice.
Another key alteration in the lung of CLP mice was the increased presence of cytokines that modulate the innate immune response against A fumigatus conidia. Mucosal sites, including the gut and lung, are constitutively Th2-promoting environments, and Dodge et al demonstrated that pulmonary DCs generate little IL-12 but are a potent source of IL-10 and IL-6.29 In the present study, it was apparent that this skewed cytokine response was further amplified by severe sepsis.15 Specifically, DCs collected from the lung of CLP mice released higher levels of IL-10 and IL-6, and lower levels of IL-12 and TNF-α, when stimulated with TLR2 and TLR4 agonists. Thus, the shift in the cytokine generation profile of DCs toward type 2 cytokines was prominent in DCs from postseptic lung.
TLR2 expression is interesting in light of previously published findings showing that TLR2 activation favors the Th2 response during allergic responses in the lung.27 We demonstrated that both lungs and DCs obtained from postseptic mice expressed more TLR2 mRNA. These data reinforce the hypothesis that DCs overtly expressing TLR2 drive a type 2 response, which may not be appropriate during the host's response to an infectious insult.
In summary, these data highlight 4 major features in the postseptic lung that may contribute to susceptibility to a secondary infection: (1) an imbalance in regulatory cytokines and chemokines within the lung; (2) diminished I-Ab expression by CD11c+ DCs; (3) a further increase in IL-10 and IL-6 generation by lung DCs; and (4) exuberant TLR2 expression in DCs and lung of CLP mice. These data are consistent with an emerging paradigm that suggests the importance of the balance of Th1 and Th2 cytokines within the lung environment, particularly in the face of ongoing pathogen challenge. The results reinforce the idea that DCs are critical cells at the interphase between the innate and acquired immune response and play a critical role in antifungal responses.
Prepublished online as Blood First Edition Paper, December 16, 2004; DOI 10.1182/blood-2004-08-3251.
Supported in part by National Institutes of Health grant nos. P50 HL60289 (S.L.K.), HL31237 (S.L.K.), and HL069865 (C.M.H.), and by a Conselho Nacional de Pesquísa e Desenvolvimiento-Brazil (CNPq) fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Robin Kunkel for her artistic assistance. We thank Holly Evanoff, Pam Lincoln, and Kristin Carpenter for their technical assistance.

![Figure 1. Survival curve, lung histopathology, and neutrophil migration of sham- or CLP-operated mice challenged or not with A fumigatus. (A) At day 15 after sham or CLP surgery, both groups of mice were challenged intratracheally with either saline or 5 × 107 A fumigatus conidia, and mouse survival was analyzed up to 7 days after challenges. Each group contained 8 to 10 mice, and data are representative of 2 independent experiments. *P < .05 between sham + Asp and CLP + Asp. (B) Pulmonary inflammatory change after A fumigatus conidia challenge in sham (i,iii,v) and CLP (ii,iv,vi) groups. At day 15 after surgery, all mice were challenged via intratracheal injection with saline or 5 × 107 conidia and were killed at day 2. The lung was collected and processed for histology. Paraffin-embedded lung sections were stained with H&E (i-ii) or GMS (iii-vi; fungal material appears black in lung sections [black dots]). Red arrows highlight dead conidia, while blue arrows highlight hyphae growth. Original magnification was × 20 for panels i-iv, and original magnification was × 100 for panels v-vi. (C) Sham and CLP surgery groups were intratracheally challenged with A fumigatus conidia at day 15 after surgery, and BAL was collected at 6 hours and 2 days after fungal challenge. The results shown are expressed as the percentage of neutrophils in the BAL, and each group contained 5 to 6 mice. The data are representative of 3 separate experiments, and are expressed as mean ± SEM. (D) Lung samples were obtained from sham- or CLP-operated mice challenged with either saline or 5 × 107 A fumigatus conidia at day 2 after challenge. The tissue was processed for MPO protein concentration assay. The data are expressed as mean ± SEM; each group contained 10 to 12 mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-08-3251/6/m_zh80090577970001.jpeg?Expires=1769608415&Signature=E1cIcRvhGPt2CLnkiQXJo29aR2O4gD7EdoQki3mBqvwohel-quIs3YTAg3ikWeziDNiRVwa4pqlUSvjUX0waw664XtppkYeq6SKvc46mrxe9BlmIi-yZNm0q0Jrr4s5Pr-gmYdZDGQXdUBan9Lpc~tfSg70LBYFke2zEAm-KfZOi~avounFT0w9A~KKJ0eGvQZKb9FXxykOAYIzqJjjhZvQl1faIRFbXyjfnT8WH-~TiN~Cy-h-~C25rcXR8cZOtoSsK-ft9wP-zwm20MaE~QZnsbeBlhGd2ciPMTgfuHLJQWPEt2UFMWTCSto~ADzSyztQuroyncEmdvvHBmf4yTw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Survival curve and lung histopathology from CLP-operated mice challenged with A fumigatus alone or coinjected with BMDCs. (A) At day 5 after CLP surgery, groups of mice were challenged intratracheally with either 5 × 107 conidia alone or coinjected with BMDCs, and mouse survival was analyzed up to 7 days after A fumigatus challenge. Each group contained 5 mice. P < .05 between CLP + Asp and CLP + Asp + BDMC. (B) At day 15 after surgery, all mice were challenged via intratracheal injection with 5 × 107 conidia alone (i) or together with 1 × 106 BMDCs obtained from 15-day sham-operated mice (ii) or obtained from 15-day CLP mice (iii). The lungs were collected at day 10 after challenge and processed for histology. Paraffin-embedded lung sections were stained with GMS (fungal material appears black in lung sections [black dots]). Original magnification was × 20 for all panels.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-08-3251/6/m_zh80090577970003.jpeg?Expires=1769608415&Signature=1GgvvSG7XnRcYKhXg1NAcySJ-MCyy-njPPhUxFizrt8F0pVDRtAH-77tDA1HwDBcYfo09upEDQRgGuGRA1GX46HQ4OXsRg9gEeLqvcsotIWm96QHvWzqF0PsOYp4j7sdzlAvyB5YgeDtww62JucMiYHYaVHA8S1PKxDqxYp30vr38Fe93yOcepu0SRgdESV4GbAQY0TEt8MVHA8y5jylA-rDxJ2BBF9pMEmpOPkFSmTArZE7kenwbnrZNBasV4TxHt-XMUE1HFja4XDtVR8bz7ld5XEzvA8nSBzfxtvCLhCaXFkh9cJUB40eU~g4GwBG6PMPutDuxcgYmHg1S3Be-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal