Abstract
In the European Organization for Research and Treatment of Cancer (EORTC) classification 2 types of primary cutaneous large B-cell lymphoma (PCLBCL) are distinguished: primary cutaneous follicle center cell lymphomas (PCFCCL) and PCLBCL of the leg (PCLBCL-leg). Distinction between both groups is considered important because of differences in prognosis (5-year survival > 95% and 52%, respectively) and the first choice of treatment (radiotherapy or systemic chemotherapy, respectively), but is not generally accepted. To establish a molecular basis for this subdivision in the EORTC classification, we investigated the gene expression profiles of 21 PCLBCLs by oligonucleotide microarray analysis. Hierarchical clustering based on a B-cell signature (7450 genes) classified PCLBCL into 2 distinct subgroups consisting of, respectively, 8 PCFCCLs and 13 PCLBCLsleg. PCLBCLs-leg showed increased expression of genes associated with cell proliferation; the proto-oncogenes Pim-1, Pim-2, and c-Myc; and the transcription factors Mum1/IRF4 and Oct-2. In the group of PCFCCL high expression of SPINK2 was observed. Further analysis suggested that PCFCCLs and PCLBCLs-leg have expression profiles similar to that of germinal center B-cell–like and activated B-cell–like diffuse large B-cell lymphoma, respectively. The results of this study suggest that different pathogenetic mechanisms are involved in the development of PCFCCLs and PCLBCLs-leg and provide molecular support for the subdivision used in the EORTC classification.
Introduction
The term primary cutaneous B-cell lymphomas refers to a heterogeneous group of B-cell neoplasms, which present in the skin without evidence of extracutaneous disease.1 A significant proportion of these primary cutaneous B-cell lymphomas are large cell lymphomas.2-5 In the European Organization for Research and Treatment of Cancer (EORTC) classification for primary cutaneous lymphomas, 2 main groups of primary cutaneous large B-cell lymphomas (PCLBCLs) are distinguished.1 Most cases are included in the group of primary cutaneous follicle center cell lymphomas (PCFCCLs). Histologically, these PCFCCLs represent a spectrum with variable proportions of (small) centrocytes and centroblasts and sometimes a follicular growth pattern in early lesions, and diffuse infiltrates of generally large centrocytes (large cleaved cells) in tumorous lesions.2-5 In the World Health Organization (WHO) classification such early lesions are classified as cutaneous follicle center lymphoma, whereas the tumorous lesions are classified as diffuse large B-cell lymphoma.6 Clinically, these PCFCCLs represent a well-defined group of primary cutaneous B-cell lymphomas, which often present with skin lesions confined to a limited skin area on the head or the trunk, rarely disseminate to extracutaneous sites, and, irrespective of the proportion of large cells or growth pattern, have an excellent prognosis (5-year survival of more than 95%).1,7 Already in the first publication on this group of PCFCCLs it was noted that patients presenting with skin tumors on the leg had a different clinical behavior.3 Recent studies demonstrated that patients with such a primary cutaneous large B-cell lymphoma of the leg (PCLBCLs-leg) differ from PCFCCLs by a higher age of onset, more frequent dissemination to extracutaneous sites, and a poorer prognosis (5-year survival of approximately 50%).7,8 Histologically, these PCLBCLs-leg show a predominance of tumor cells with round nuclei and, in contrast to the group of PCFCCLs, strongly express Bcl-2 protein.7-10 For these reasons these PCLBCLs-leg were included as a separate entity in the EORTC classification.1 Distinction between these 2 types of PCLBCLs is clinically important, since it dictates the first choice of treatment: radiotherapy in PCFCCLs and anthracyclinebased chemotherapy in PCLBCLs-leg.7 By following the WHO classification, both groups will be lumped together in the group of diffuse large B-cell lymphoma, and all patients will be treated with anthracycline-based chemotherapy with or without radiotherapy. Although recent studies11-13 confirmed that these PCLBCLs-leg are a distinct group with an intermediate prognosis, the subdivision of primary cutaneous B-cell lymphomas into 2 main categories (PCLBCLs-leg and PCFCCLs) primarily based on site of presentation (leg versus other sites) has been much disputed.14
Recent studies have started to evaluate the genetic mechanisms involved in the development and progression of these lymphomas. However, the number of studies published to date is still limited, and specific cytogenetic abnormalities have not been identified yet. Most studies have focused on the differential expression of the Bcl-2 protein in the different groups of PCLBCLs. Most authors agree that the Bcl-2 overexpression in the group of PCLBCLs-leg is not associated with the t(14;18) translocation as observed in most follicular and some diffuse large B-cell lymphomas in lymph nodes.9,14-16 In some cases it might result from chromosomal amplification of the BCL-2 gene.17,18 Recent comparative genomic hybridization (CGH) studies demonstrated 6q loss and 18q gain in a proportion of PCLBCLs, but not in PCFCCLs.18 Inactivation of p15 and p16 tumor suppressor genes by promoter hypermethylation has been detected in 11% and 44% of PCLBCLs, respectively.19
In the present study we performed oligonucleotide microarray analysis on a large group of PCLBCLs, including 8 PCFCCLs and 13 PCLBCLs-leg. The main purpose of this study was to find out if the 2 types of PCLBCLs recognized in the EORTC classification have different gene expression profiles. Differences in the gene expression profiles might not only give insight in the pathogenetic mechanisms underlying the differences in clinical behavior between the 2 types of PCLBCLs, but they could also provide a rationale for future classifications of these PCLBCLs and result in the identification of genes and pathways that might serve as diagnostic or prognostic markers or as potential targets for therapeutic intervention.
Patients, materials, and methods
Patient selection
Pretreatment skin biopsies from 8 patients with PCFCCL with a diffuse large cell histology and 13 patients with PCLBCL-leg were included in this study. Only cases in which large neoplastic B cells constituted 80% or more of the total number of infiltrating cells were selected. The diagnosis PCFCCL or PCLBCL-leg was based on the criteria of the EORTC classification.1 Using the WHO classification all cases were classified as diffuse large B-cell lymphoma.6 In all patients there was no evidence of extracutaneous disease at time of diagnosis as assessed by adequate staging procedures including physical examination, complete blood cell counts, computed tomography of chest and abdomen, and bone marrow biopsy.
Patients with a PCFCCL presented with localized skin lesions either on the scalp (5 cases) or trunk (3 cases). Histologically, 7 cases showed a diffuse proliferation of predominantly large cleaved cells (large centrocytes), whereas in one case (no. 1) almost equal numbers of large cleaved cells and large noncleaved cells (centroblasts) were observed. In one case (no. 6) small clusters of CD35+ follicular dendritic cells were seen, suggesting a preceding follicular growth pattern. Immunostaining showed 15% to 20% (median, 20%) admixed T cells and few scattered CD68+ macrophages. Consistent with previous studies of our group, the neoplastic B cells were either completely negative for Bcl-2 protein or showed a weak staining on a minor proportion (< 20%) of tumor cells.
The 13 patients with a PCLBCL-leg presented with skin tumors on one (11 cases) or both legs (2 cases) (Table 1). One patient presented with an additional skin tumor on the right cheek (no. 9). Histologically, all cases showed diffuse infiltrates of centroblasts and immunoblasts, and in 2 cases (no. 17 and no. 18) a considerable admixture (ca 30%) of large cleaved cells. The numbers of admixed T cells varied between 5% and 20% (median, 10%) and was on the average much lower than in the group of PCFCCLs. Bcl-2 protein was strongly expressed by the large majority of tumor cells in 11 of 13 cases. In the other 2 cases (no. 13 and no. 18) Bcl-2 was expressed by 40% and fewer than 10% of the neoplastic B cells, respectively.
Clinical characteristics of patients with primary cutaneous large B-cell lymphoma
Case no. . | Clinical presentation . | Sex (age at diagnosis, y) . | Initial therapy . | Result of initial therapy . | Relapse . | Follow-up status, (period, mo) . |
|---|---|---|---|---|---|---|
| PCFCCL | ||||||
| 1 | Multiple tumors on the scalp | M (76) | RT | CR | None | D- (36) |
| 2 | Localized tumors on the chest | M (38) | RT | CR | None | A- (192) |
| 3 | Localized plaques and tumors on the back | M (62) | RT | CR | Skin + EC | D- (41) |
| 4 | Grouped plaques and tumors on the scalp | M (61) | RT | CR | Skin | A- (109) |
| 5 | Solitary tumor on the scalp | F (69) | RT | CR | None | A- (127) |
| 6 | Solitary tumor on the scalp | M (37) | RT | CR | Skin | A- (68) |
| 7 | Grouped plaques and tumors on the chest | F (50) | RT | CR | None | A- (24) |
| 8 | Solitary tumor on the scalp | M (55) | RT | CR | None | A- (8) |
| PCLBCL-leg | ||||||
| 9 | Tumors on right cheek and right upper leg | M (69) | CHOP | CR | Skin + EC | D+ (35) |
| 10 | 3 tumors on left lower leg | M (47) | CHOP | CR | None | A- (32) |
| 11 | Multiple tumors on right lower leg | F (84) | CHOP | CR | Skin | A- (30) |
| 12 | 2 tumors on left lower leg | F (74) | CHOP | CR | None | A- (30) |
| 13 | Solitary tumor on lower leg | F (62) | RT | CR | None | D- (36) |
| 14 | Multiple tumors on left lower leg | M (89) | CHOP | CR | Skin + EC | D+ (12) |
| 15 | Multiple tumors on left lower leg | F (90) | PUVA-phototherapy | PR | Skin + EC | D+ (29) |
| 16 | Solitary tumor on left lower leg | F (88) | RT | CR | Skin + EC | D+ (26) |
| 17 | Tumors and plaque on right lower leg | F (77) | RT | CR | Skin | A- (45) |
| 18 | Solitary tumor on left lower leg | F (83) | RT | CR | EC | D+ (24) |
| 19 | Multiple tumors on both legs | F (76) | CHOP | CR | Skin | D- (11) |
| 20 | Multiple tumors on both lower legs | F (75) | CHOP | CR | None | A- (12) |
| 21 | Solitary tumor on right lower leg | F (75) | RT | CR | Skin + EC | D+ (73) |
Case no. . | Clinical presentation . | Sex (age at diagnosis, y) . | Initial therapy . | Result of initial therapy . | Relapse . | Follow-up status, (period, mo) . |
|---|---|---|---|---|---|---|
| PCFCCL | ||||||
| 1 | Multiple tumors on the scalp | M (76) | RT | CR | None | D- (36) |
| 2 | Localized tumors on the chest | M (38) | RT | CR | None | A- (192) |
| 3 | Localized plaques and tumors on the back | M (62) | RT | CR | Skin + EC | D- (41) |
| 4 | Grouped plaques and tumors on the scalp | M (61) | RT | CR | Skin | A- (109) |
| 5 | Solitary tumor on the scalp | F (69) | RT | CR | None | A- (127) |
| 6 | Solitary tumor on the scalp | M (37) | RT | CR | Skin | A- (68) |
| 7 | Grouped plaques and tumors on the chest | F (50) | RT | CR | None | A- (24) |
| 8 | Solitary tumor on the scalp | M (55) | RT | CR | None | A- (8) |
| PCLBCL-leg | ||||||
| 9 | Tumors on right cheek and right upper leg | M (69) | CHOP | CR | Skin + EC | D+ (35) |
| 10 | 3 tumors on left lower leg | M (47) | CHOP | CR | None | A- (32) |
| 11 | Multiple tumors on right lower leg | F (84) | CHOP | CR | Skin | A- (30) |
| 12 | 2 tumors on left lower leg | F (74) | CHOP | CR | None | A- (30) |
| 13 | Solitary tumor on lower leg | F (62) | RT | CR | None | D- (36) |
| 14 | Multiple tumors on left lower leg | M (89) | CHOP | CR | Skin + EC | D+ (12) |
| 15 | Multiple tumors on left lower leg | F (90) | PUVA-phototherapy | PR | Skin + EC | D+ (29) |
| 16 | Solitary tumor on left lower leg | F (88) | RT | CR | Skin + EC | D+ (26) |
| 17 | Tumors and plaque on right lower leg | F (77) | RT | CR | Skin | A- (45) |
| 18 | Solitary tumor on left lower leg | F (83) | RT | CR | EC | D+ (24) |
| 19 | Multiple tumors on both legs | F (76) | CHOP | CR | Skin | D- (11) |
| 20 | Multiple tumors on both lower legs | F (75) | CHOP | CR | None | A- (12) |
| 21 | Solitary tumor on right lower leg | F (75) | RT | CR | Skin + EC | D+ (73) |
PCFCCL indicates primary cutaneous follicle center cell lymphoma; PCLBCL-leg, primary cutaneous large B-cell lymphoma of the leg; CHOP, multiagent anthracycline-based chemotherapy; RT, radiotherapy; CR, complete remission; PR, partial remission; EC, extracutaneous relapse; A -, alive with no evidence of disease; A +, alive with disease; D+, died of lymphoma; D-, died of other cause.
This study was performed in accordance with the Dutch code and Leiden University Medical Center guidelines on leftover material.
Oligonucleotide microarrays
Samples and microarrays were processed according to the manufacturer's protocol (available from Affymetrix, Santa Clara, CA). In brief, on average between 20 and 60 μg RNA was isolated from 50 × 20 μm frozen sections with the RNeasy-kit (Qiagen, Hilden, Germany). Using the MessageAmp aRNA kit (Ambion, Huntingdon, United Kingdom), total RNA was reverse-transcribed using an oligo(dT)-T7 promoter primer to prime first-strand synthesis. After second-strand synthesis, the purified cDNA product was in vitro transcribed using T7 RNA polymerase, biotin-UTP (biotin-5′-triphosphate), and biotin-CTP (cytidine triphosphate) to generate fragmented biotinylated aRNA. Fragmented aRNA (15 μg) was hybridized to a Human Genome U95Av2 Array (Affymetrix), interrogating 12 625 human transcripts for 16 hours at 45°C with constant rotation at 60 rpm. After hybridization, the microarray was washed, stained on an Affymetrix fluidics station, and scanned with an argon-ion confocal laser with 488-nm excitation and 570-nm detection wavelengths.
Data processing and analysis
The array images were quantified using the MicroArray Suite (MAS) v 5.0 software (Affymetrix). The average fluorescence intensity was determined for each microarray, and then the output of each experiment was globally scaled to a target value of 200. Normalization of the data were performed using variant stability and normalization (VSN, Stanford, CA), part of the R statistical software package (available at www.bioconductor.org).20 Gene expression patterns were further analyzed using Spotfire DecisionSite (Spotfire, Goteborg, Sweden) and Statistical Analysis of Microarrays (SAM, Stanford, CA).21 First, unsupervised hierarchical clustering (ie, based on the expression of all genes present at the microarray) was performed using Spotfire DecisionSite. To correct for potential influences of the tumor microenvironment on expression data, a B-cell signature was generated to focus specifically on the genes expressed by the tumor cells. This B-cell signature consisted of all 7450 genes with a “present” call in the datasets of at least 1 of 4 normal B-cell subsets (centroblasts, centrocytes, memory B cells, and naive B cells) generated by the same Affymetrix U95Av2 Gene Chip (more information about the normal subsets are in the paper of Klein et al.22 ) To further investigate differentially expressed genes in both subgroups, the supervised comparison analysis technique Statistical Analysis of Microarrays (SAM) was applied to compare gene expression patterns of PCFCCL and PCLBCL-leg. A false discovery rate of less than 1.0 was chosen to select genes that were significantly up- or down-regulated. Gene expression data were transformed into z-scores as described previously, and obtained output was visualized using Spotfire DecisionSite.23
Quantitative real-time PCR
Expression values of 6 differentiating genes between PCFCCL and PCLBCL-leg identified by microarray and Bcl-2 alpha and beta splice variants were analyzed by quantitative polymerase chain reaction (qPCR) in 4 samples from PCFCCLs and 4 samples from PCLBCLs-leg. cDNA synthesis was performed on 1 μg total RNA after treatment with RQ1 DNase I (Promega, Madison, WI) using Superscript III reverse transcriptase (Invitrogen, Breda, The Netherlands) and an oligo(dT)12-18 primer (Invitrogen) in a final volume of 20 μL. Real-time PCR was performed with the ABI-Prism 7700 instrument and the SYBR Green PCR Master Mix (Applied Biosystems, Nieuwerkerk aan den IJssel, The Netherlands). The cycle parameters for these transcripts and for the housekeeping genes U1A and RPS11 used for normalization were as follows: denaturing for 15 seconds at 95°C; annealing and extension for 60 seconds at 60°, for 40 cycles. The primer sequences (Invitrogen) of selected transcripts are given in Table 2. For analysis of BCL2 expression primer combinations detecting 2 different splice variants (BCL2 alpha and BCL2 beta) were used.24 Data were evaluated using the sequence detection software (SDS) version 1.9.1 (Applied Biosystems) and the second derivative maximum algorithm. Specificity of the PCR product was confirmed by agarose gel electrophoresis and subsequent DNA sequence analysis of test samples and melting curve analysis in the case of patient material. Serial dilutions of cDNA synthesized from different cell lines were used to generate the standard curves for the primer combinations.
Primers sequences and accession numbers of selected transcripts quantified by real-time PCR
Gene . | Accession no. . | 5′ primer . | 3′ primer . |
|---|---|---|---|
| PIM1 | NM_002648 | CCAGCAAATAGCAGCCTTTC | GTCACTGGTACTCGGGAAGC |
| PIM2 | NM_006875 | TTGGGAAGGAATGGAAGATG | TTATTTCCCCTAGCCCATCC |
| Cyclin E | NM_001238 | AGCGGTAAGAAGCAGAGCAG | CGCTGCAACAGACAGAAGAG |
| OCT2 | NM_002698 | AGAGGAGATCCTGCTGATCG | GGTTGATGCGTTTCTCCTTC |
| CMYC | NM_002467 | AGATCCCGGAGTTGGAAAAC | AGCTTTTGCTCCTCTGCTTG |
| MUM1/IRF4 | NM_002460 | TTACCACCAAGGGCAGGTAG | ACCCAAGACTCCCACAGTTG |
| BCL2 alpha | NM_000633 | GCCCTGTGGATGACTGAGTA | GGCCGTACAGTTCCACAAAG |
| BCL2 beta | NM_000657 | GCCCTGTGGATGACTGAGTA | ATCACCAGATGCACCTACCC |
Gene . | Accession no. . | 5′ primer . | 3′ primer . |
|---|---|---|---|
| PIM1 | NM_002648 | CCAGCAAATAGCAGCCTTTC | GTCACTGGTACTCGGGAAGC |
| PIM2 | NM_006875 | TTGGGAAGGAATGGAAGATG | TTATTTCCCCTAGCCCATCC |
| Cyclin E | NM_001238 | AGCGGTAAGAAGCAGAGCAG | CGCTGCAACAGACAGAAGAG |
| OCT2 | NM_002698 | AGAGGAGATCCTGCTGATCG | GGTTGATGCGTTTCTCCTTC |
| CMYC | NM_002467 | AGATCCCGGAGTTGGAAAAC | AGCTTTTGCTCCTCTGCTTG |
| MUM1/IRF4 | NM_002460 | TTACCACCAAGGGCAGGTAG | ACCCAAGACTCCCACAGTTG |
| BCL2 alpha | NM_000633 | GCCCTGTGGATGACTGAGTA | GGCCGTACAGTTCCACAAAG |
| BCL2 beta | NM_000657 | GCCCTGTGGATGACTGAGTA | ATCACCAGATGCACCTACCC |
Immunohistochemistry
From 5 PCFCCLs and 11 PCLBCLs-leg paraffin material was still available for additional immunohistochemical stainings with antibodies against Mum1/IRF4 and the proliferation marker Ki-67. Staining was performed on formalin-fixed, paraffin-embedded serial sections of the same tumors analyzed by oligonucleotide microarray using standard procedures. After antigen retrieval by boiling for 10 minutes in 10 mM citrate buffer (pH 6.0) for Ki-67 and in 1.0 mM EDTA (ethylenediaminetetraacetic acid buffer) (pH 9.0) for Mum1/IRF4, tissue sections were incubated overnight with antibodies against respectively Ki-67 (clone MIB-1, 1:400, DAKO, Glostrup, Denmark) and against Mum1/IRF4 (1:100; antibody was a kind gift of Professor G. Cattoretti, Institute for Cancer Genetics, Columbia University, New York, NY). Sections were then incubated with biotin-labeled rabbit antimouse antibodies (1:200). Immunoreactivity was detected using a streptavidin-biotin-peroxidase complex (sABC-HRP, DakoCytomation K0377; 1:100; DAKO). Subsequently, a 10-minute incubation with diaminobenzidine (DAB) solution (Sigma-Aldrich, Zwijndrecht, The Netherlands) was performed. All secondary and tertiary antibodies were incubated for 30 minutes in 1% phosphate-buffered saline (PBS)/bovine serum albumin (BSA) at room temperature.
Results
PCFCCLs and PCLBCLs-leg show distinct profiles of B-cell–expressed genes
The microarray analysis of all tumor samples showed an average expression of 55% (range, 52.1%-58.8%) of all the 12 625 genes represented on the oligonucleotide array. In the group of, respectively, PCFCCLs and PCLBCLs-leg, an average number of 7098 and 7086 transcripts were present.
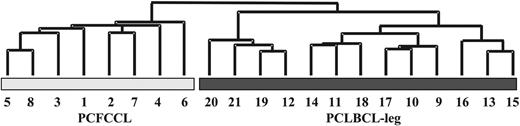
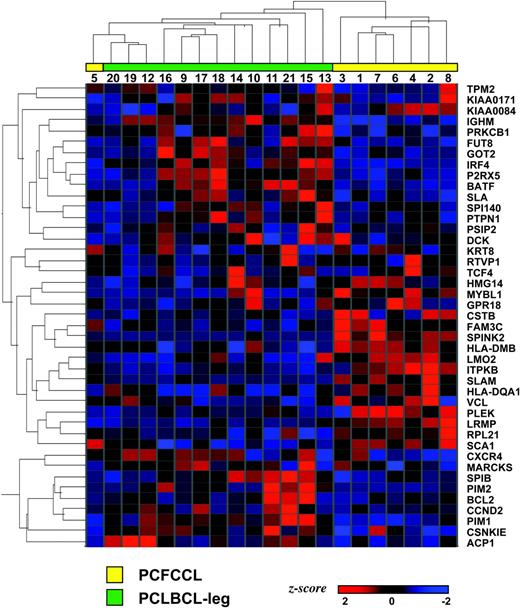
Unsupervised hierarchical clustering on the basis of all expressed genes separated 8 PCFCCLs and 10 PCLBCLs-leg in different groups, whereas 3 PCLBCL-leg (cases 12, 19, and 20) were clustered in the PCFCCL group (data not shown). However, unsupervised hierarchical clustering based on 7450 B-cell signature genes (“Patients, materials, and methods”) classified the 21 PCLBCLs into 2 distinct subgroups consisting of 8 PCFCCL and 13 PCLBCL-leg as shown by the dendrogram in Figure 1.
Hierarchical clustering based on B-cell signature in primary cutaneous large B-cell lymphoma. Hierarchical clustering based on the expression of a B-cell signature consisting of 7450 genes with a “present” call in normal B-cell subsets (centroblasts, centrocytes, memory B cells, and naive B cells) and generated by the same Affymetrix U95Av2 Gene Chip22 identifies 2 distinct gene expression profiles in primary cutaneous follicle center cell lymphoma (PCFCCL) and primary cutaneous large B-cell lymphoma of the leg (PCLBCL-leg).
Hierarchical clustering based on B-cell signature in primary cutaneous large B-cell lymphoma. Hierarchical clustering based on the expression of a B-cell signature consisting of 7450 genes with a “present” call in normal B-cell subsets (centroblasts, centrocytes, memory B cells, and naive B cells) and generated by the same Affymetrix U95Av2 Gene Chip22 identifies 2 distinct gene expression profiles in primary cutaneous follicle center cell lymphoma (PCFCCL) and primary cutaneous large B-cell lymphoma of the leg (PCLBCL-leg).
Differentially expressed genes in PCLBCLs
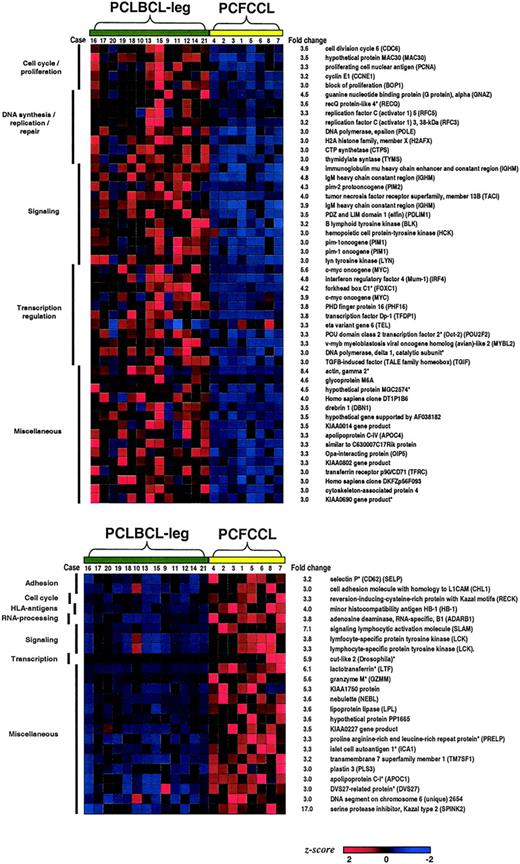
Expression profiles of the entire set of genes of the 8 PCFCCL samples and the 13 PCLBCL-leg samples were compared using the significance analysis of microarrays (SAM) technique to identify most differentiating genes between both groups. In this analysis all 12 625 genes were included in order not to miss genes aberrantly expressed by tumor cells or genes that are expressed in late stages of B-cell differentiation (eg, plasma cells), which are not covered by the generated B-cell signature. Seventy-one statistically significant differentially expressed genes at P less than .01 were identified as most discriminating genes between PCFCCL and PCLBCL-leg with fold changes of at least 3.0. Of these 71 most discriminating genes between PCFCCL and PCLBCL-leg, 48 genes were highly expressed in the group of PCLBCLs-leg, and 23 genes were up-regulated in the group of PCFCCLs as visualized in Figure 2.
Differentiating genes in primary cutaneous large B-cell lymphoma. Seventy-one statistically significant differentially expressed genes identified as most discriminating genes between primary cutaneous follicle center cell lymphoma (PCFCCL) and primary cutaneous large B-cell lymphoma of the leg (PCLBCL-leg) with fold changes of at least 3.0 were classified by biologic function. Forty-eight genes were highly expressed in the group of PCLBCL-leg, and 23 genes were up-regulated in the group of PCFCCL. Genes indicated by an asterisk (*) were not present in the B-cell signature.22
Differentiating genes in primary cutaneous large B-cell lymphoma. Seventy-one statistically significant differentially expressed genes identified as most discriminating genes between primary cutaneous follicle center cell lymphoma (PCFCCL) and primary cutaneous large B-cell lymphoma of the leg (PCLBCL-leg) with fold changes of at least 3.0 were classified by biologic function. Forty-eight genes were highly expressed in the group of PCLBCL-leg, and 23 genes were up-regulated in the group of PCFCCL. Genes indicated by an asterisk (*) were not present in the B-cell signature.22
Clustering by biologic function showed that several of the 48 genes highly expressed in the group of PCLBCLs were associated with cellular proliferation. These included cell-cycle genes, such as cyclin E, CDC6, and proliferating cell nuclear antigen (PCNA); genes involved in DNA synthesis and repair, such as CTP synthetase, DNA polymerase, and replication factors 3 and 5; and genes encoding transcription factors regulating cellular proliferation including DP1, CMYC, and MYBL2. Other genes highly up-regulated in the PCLBCLs-leg group were IgM heavy chain and related genes involved in Ig-mediated B-cell signaling, such as lyn and blk and the B-cell transcription factors MUM1/IRF4 and OCT2, as well as the genes encoding the Pim kinases (PIM1 and PIM2). As illustrated in Figure 2, the degree of up-regulation of most individual genes varied between individual samples of PCLBCLs-leg. The most consistently overexpressed genes in almost all cases of the PCLBCLs-leg group were IGM heavy chain (13 of 13 cases), PIM1 (12 of 13 cases), and MUM1/IRF4 (12 of 13 cases).
Of the 23 genes highly expressed in the group of PCFCCLs, no evident clusters of genes with similar biologic function or involved in common pathways could be identified. However, the most prominent distinction between PCFCCLs and PCLBCLs-leg came from SPINK2, which was highly expressed in all 8 PCFCCL samples with a fold change of 17.0. In addition, several genes associated with the reactive antitumor immune response were substantially up-regulated in the group of PCFCCL, including granzyme M selectively expressed by NK cells and the T-cell–expressed genes selectin P and LCK, which may reflect the higher number of admixed inflammatory cells in our group of PCFCCLs.
Gene expression of PCLBCLs confirmed by quantitative real-time PCR
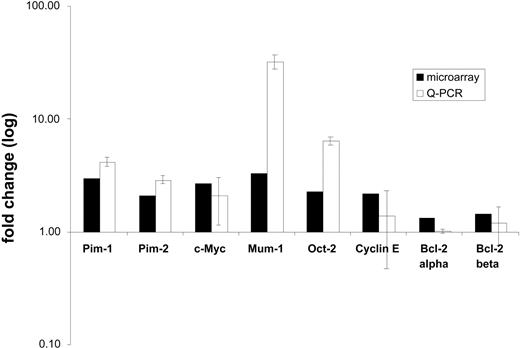
To validate the results of microarray analysis, quantitative real-time PCR (qPCR) was applied on a panel of 7 genes including CMYC, PIM1, PIM2, MUM1/IRF4, OCT2, cyclin E, and 2 splice variants of BCL2 (BCL2 alpha and beta). The fold changes in expression between PCFCCLs and PCLBCLs-leg resulting from qPCR analysis were calculated and compared with the fold changes obtained by microarray analysis. Except for cyclin E, qPCR results all were in agreement with oligonucleotide array results as shown in Figure 3. No statistically significant differences in BCL2 mRNA expression were found by qPCR between the PCFCCLs and PCLBCLsleg groups, although the signals for the BCL2 beta splice variant were slightly higher in the group of PCLBCLs-leg. These qPCR measurements are in line with oligonucleotide array results as shown in Figure 3.
Results of quantitative real-time PCR analysis (qPCR). Histogram showing expression of 6 selected differentially expressed genes and BCL2 alpha and beta splice variants as measured by oligonucleotide microarray analysis (▪) and in 4 primary cutaneous follicle center cell lymphoma and 4 primary cutaneous large B-cell lymphoma of the leg by qPCR (□). Fold changes of oligonucleotide microarray and qPCR experiments derived data denote average expression level in patients relative to each other. A fold change of 1 indicates equal expression in both groups. The error bars represent standard deviations of the mean expression values as measured by qPCR.
Results of quantitative real-time PCR analysis (qPCR). Histogram showing expression of 6 selected differentially expressed genes and BCL2 alpha and beta splice variants as measured by oligonucleotide microarray analysis (▪) and in 4 primary cutaneous follicle center cell lymphoma and 4 primary cutaneous large B-cell lymphoma of the leg by qPCR (□). Fold changes of oligonucleotide microarray and qPCR experiments derived data denote average expression level in patients relative to each other. A fold change of 1 indicates equal expression in both groups. The error bars represent standard deviations of the mean expression values as measured by qPCR.
Immunohistochemistry
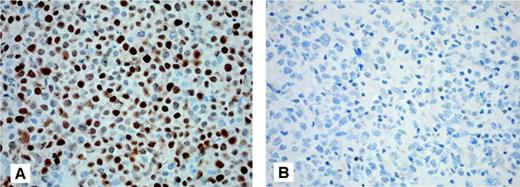
Because the MUM1/IRF4 transcript was consistently up-regulated in the group of PCLBCLs-leg, Mum1/IRF4 protein expression was investigated by immunohistochemistry in 11 PCLBCLs-leg and 5 PCFCCLs. Tumor cells 8 of 11 PCLBCLs-leg showed strong expression of Mum1/IRF4 (> 80% positive-staining cells) as shown in Figure 4A. In the other 3 cases 30% (no. 10 and no. 19) to 50% (no. 12) of the tumor cells were Mum1/IRF4-positive. In contrast, the tumor cells of all 5 PCFCCL were Mum1/IRF4-negative (10% or less positive staining cells), as shown in Figure 4B. There was a good correlation between Mum1/IRF4 protein and mRNA expression by microarray array with mean absolute expression value of MUM1/IRF4 of 322 (range, 172-437) for the 8 PCFCCL and 1570 (range, 397-2637) for the 13 PCLBCL-leg. Although Mum1/IRF4 protein expression has been reported to have prognostic significance in B-CLL, a relationship between Mum1/IRF4 expression and clinical outcome was not present in PCLBCLs-leg.25,26
Expression of Mum1/IRF4 in primary cutaneous large B-cell lymphoma. Neoplastic B cells of primary cutaneous large B-cell lymphoma of the leg show a positive staining for Mum1/IRF4 (A), whereas neoplastic cells of primary cutaneous follicle center cell lymphoma are Mum1/IRF4-negative (B) (original magnification × 200; Zeiss Axioshop 2 plus). Images were captured with a Leica DM6000B microscope (Leica, Rijswijk, The Netherlands), using an HCPlan APO objective lens (200×/0.70) and a ProgRes C10 camera with ProgRes10 software (JenaOptik, Jena, Germany).
Expression of Mum1/IRF4 in primary cutaneous large B-cell lymphoma. Neoplastic B cells of primary cutaneous large B-cell lymphoma of the leg show a positive staining for Mum1/IRF4 (A), whereas neoplastic cells of primary cutaneous follicle center cell lymphoma are Mum1/IRF4-negative (B) (original magnification × 200; Zeiss Axioshop 2 plus). Images were captured with a Leica DM6000B microscope (Leica, Rijswijk, The Netherlands), using an HCPlan APO objective lens (200×/0.70) and a ProgRes C10 camera with ProgRes10 software (JenaOptik, Jena, Germany).
To investigate proliferation rates of PCLBCLs, paraffin sections of the same tumors also were stained with an antibody against the proliferation marker Ki-67. Whereas Ki-67 was expressed by more than 75% of the tumor cells in all PCLBCLs-leg, Ki-67 staining in PCFCCLs was much more variable with percentages varying between 10% and 80% (median, 35%).
Activated B-cell profile versus germinal center B-cell profile
Several of the highly expressed genes in the PCLBCLs-leg group including genes encoding for IgM heavy chain, MUM1/IRF4, and both Pim-kinases (PIM1 and PIM2) were recognized as members of the activated B-cell (ABC) profile identified by microarray analysis in a subset of nodal diffuse large B-cell lymphomas (DLBCLs) characterized by an aggressive clinical behavior.27 Likewise a number of genes with high expression in PCFCCLs, including SPINK2, LCK, and SLAM, were included in the germinal center B-cell (GCB) profile corresponding to a subset of DLBCLs with a more favorable clinical outcome. To test the hypothesis that PCLBCLs-leg are related to ABC-like DLBCL and PCFCCL to GCB-like DLBCL, we used a recently described list consisting of 43 genes that discriminate both subgroups of DLBCL with highest significance.28 These 43 genes were identified previously using the Affymetrix HU6500 microarray after the assignation of 274 DLBCL to ABC-type or GCB-type using a predictor model based on 14 genes represented on the Affymetrix HU6500 microarray of 27 predictor genes of the lymphochip. All above-mentioned genes, except LCK, were selected in this list as well.
Hierarchical clustering based on the selected subset of these 43 genes clustered 7 of 8 PCFCCL and all 13 PCLBCL-leg, together suggesting a similarity in gene expression profiles between PCLBCL-leg and ABC-like DLBCL and between PCFCCL and GCB-like DLBCL, respectively (Figure 5).
Expression of activated B-cell profile and germinal center profile genes in primary cutaneous large B-cell lymphoma. Hierarchical clustering analysis of primary cutaneous follicle center cell lymphoma (PCFCCL) and primary cutaneous large B-cell lymphoma of the leg (PCLBCL-leg) was performed based on the expression of 43 genes present in a recently described list consisting of 43 genes that discriminate between activated B-cell (ABC) and germinal center B-cell (GCB) types of diffuse large B-cell lymphoma with highest significance.27 These 43 genes were identified previously using the Affymetrix HU6500 microarray after the assignation of 274 DLBCL to ABC type or GCB type using a predictor model based on 14 genes represented on the Affymetrix HU6500 microarray of 27 predictor genes of the lymphochip. Hierarchical clustering based on these 43 selected genes clustered, respectively, 7 of 8 PCFCCL and 13 of 13 PCLBCL-leg in 2 distinct groups.
Expression of activated B-cell profile and germinal center profile genes in primary cutaneous large B-cell lymphoma. Hierarchical clustering analysis of primary cutaneous follicle center cell lymphoma (PCFCCL) and primary cutaneous large B-cell lymphoma of the leg (PCLBCL-leg) was performed based on the expression of 43 genes present in a recently described list consisting of 43 genes that discriminate between activated B-cell (ABC) and germinal center B-cell (GCB) types of diffuse large B-cell lymphoma with highest significance.27 These 43 genes were identified previously using the Affymetrix HU6500 microarray after the assignation of 274 DLBCL to ABC type or GCB type using a predictor model based on 14 genes represented on the Affymetrix HU6500 microarray of 27 predictor genes of the lymphochip. Hierarchical clustering based on these 43 selected genes clustered, respectively, 7 of 8 PCFCCL and 13 of 13 PCLBCL-leg in 2 distinct groups.
Discussion
In the EORTC classification of cutaneous lymphomas, 2 types of PCLBCLs are recognized: PCFCCL and PCLBCL-leg. The clinical, histologic, immunophenotypical, and genetic differences between both groups are summarized in Table 3. Although recent studies have provided further support for the clinical significance of this classification, there is still ongoing debate regarding the subdivision of PCLBCLs into 2 main categories primarily based on site of presentation.18-20 Understanding of the genetic mechanisms involved in the development and progression of these lymphomas might provide a solution for this controversy.
Clinical, histologic, immunophenotypic, and genetic differences between PCFCCL with a diffuse large cell histology and PCLBCL-leg
. | PCFCCL, diffuse large cell . | PCLBCL-leg . | References . |
|---|---|---|---|
| Clinical features | |||
| Median age at diagnosis (range) | 62 y (14-88 y) | 75 y (27-92 y) | Grange et al,7 Vermeer et al,8 Geelen et al,9 Goodlad et al11 |
| Male-female ratio | 1:1 | 3:1 | Grange et al,7 Vermeer et al,8 Goodlad et al11 |
| Site | Trunk; head (scalp) | Leg(s) | Willemze et al1 |
| Treatment of first choice | Radiotherapy | Systemic chemotherapy | Willemze et al,1 Grange et al7 |
| Dissemination | 11% | 50% | Grange et al,7 Vermeer et al8 |
| 5-year survival rate | > 95% | 52% | Grange et al,7 Vermeer et al8 |
| Histopathology | |||
| Cytomorphology | Large cleaved (85%) | Round (90%) | Willemze et al,3 Berti et al,4 Santucci et al,5 Grange et al,7 Vermeer et al8 |
| Blastic transformation | Often* | No (de novo) | Willemze et al,3 Berti et al,4 Santucci et al,5 Grange et al,7 Vermeer et al8 |
| Bcl-2 expression | -/+† | + | Geelen et al,9 Hoefnagel et al,10 Goodlad et al11 |
| Bcl-6 expression | + | +/- | Hoefnagel et al,10 Goodlad et al,11 Paulli et al12 |
| CD10 expression | - | - | Hoefnagel et al,10 Paulli et al12 |
| Mum1/IRF4 expression | - | + | Present study, Paulli et al12 |
| Genetics | |||
| t(14; 18) | Absent | Absent | Geelen et al,9 Child et al,15 Cerroni et al,16 Hallermann et al18 |
| CGH analysis | |||
| loss 6q | 0 of 9 | 4 of 13 | Hallermann et al18 |
| gain 18q | 0 of 9 | 8 of 13 | Hallermann et al18 |
| FISH analysis | |||
| IGH breakpoint | 0 of 6 | 7 of 14 | Sánchez-Beato et al29 |
| CMYC breakpoint | 0 of 6 | 6 of 14 | Sánchez-Beato et al29 |
| BCL6 breakpoint | 0 of 6 | 5 of 14 | Sánchez-Beato et al29 |
| Gene expression profile | Germinal center B-cell type (GCB) | Activated B-cell type (ABC) | Present study |
. | PCFCCL, diffuse large cell . | PCLBCL-leg . | References . |
|---|---|---|---|
| Clinical features | |||
| Median age at diagnosis (range) | 62 y (14-88 y) | 75 y (27-92 y) | Grange et al,7 Vermeer et al,8 Geelen et al,9 Goodlad et al11 |
| Male-female ratio | 1:1 | 3:1 | Grange et al,7 Vermeer et al,8 Goodlad et al11 |
| Site | Trunk; head (scalp) | Leg(s) | Willemze et al1 |
| Treatment of first choice | Radiotherapy | Systemic chemotherapy | Willemze et al,1 Grange et al7 |
| Dissemination | 11% | 50% | Grange et al,7 Vermeer et al8 |
| 5-year survival rate | > 95% | 52% | Grange et al,7 Vermeer et al8 |
| Histopathology | |||
| Cytomorphology | Large cleaved (85%) | Round (90%) | Willemze et al,3 Berti et al,4 Santucci et al,5 Grange et al,7 Vermeer et al8 |
| Blastic transformation | Often* | No (de novo) | Willemze et al,3 Berti et al,4 Santucci et al,5 Grange et al,7 Vermeer et al8 |
| Bcl-2 expression | -/+† | + | Geelen et al,9 Hoefnagel et al,10 Goodlad et al11 |
| Bcl-6 expression | + | +/- | Hoefnagel et al,10 Goodlad et al,11 Paulli et al12 |
| CD10 expression | - | - | Hoefnagel et al,10 Paulli et al12 |
| Mum1/IRF4 expression | - | + | Present study, Paulli et al12 |
| Genetics | |||
| t(14; 18) | Absent | Absent | Geelen et al,9 Child et al,15 Cerroni et al,16 Hallermann et al18 |
| CGH analysis | |||
| loss 6q | 0 of 9 | 4 of 13 | Hallermann et al18 |
| gain 18q | 0 of 9 | 8 of 13 | Hallermann et al18 |
| FISH analysis | |||
| IGH breakpoint | 0 of 6 | 7 of 14 | Sánchez-Beato et al29 |
| CMYC breakpoint | 0 of 6 | 6 of 14 | Sánchez-Beato et al29 |
| BCL6 breakpoint | 0 of 6 | 5 of 14 | Sánchez-Beato et al29 |
| Gene expression profile | Germinal center B-cell type (GCB) | Activated B-cell type (ABC) | Present study |
May sometimes be preceded by PCFCCL with a predominance of small centrocytes and/or follicular growth pattern
May be weakly positive in a minority of tumor cells
In the present study gene expression profiles of 8 PCFCCLs with a diffuse large cell histology and 13 PCLBCLs-leg were generated using Affymetrix oligonucleotide arrays. Only cases in which large neoplastic B cells constituted 80% or more of the total number of infiltrating cells were included in this study. Hierarchical clustering based on 7450 genes expressed in a created B-cell signature (“Patients, materials, and methods”), used to minimize the effect of nonmalignant cells present in the skin biopsies, classified the 21 PCLBCLs into 2 distinct subgroups consisting of 8 PCFCCLs and 13 PCLBCLs-leg. Analysis of the expression of 43 genes recently described to discriminate the ABC and GCB types of DLBCLs with highest significance suggested a similarity between PCLBCL-leg and ABC-like DLBCL and PCFCCL and GCB-like DLBCL, respectively.28 These observations do not only provide significant support for the subdivision used in the EORTC classification, but also suggest that different pathogenetic mechanisms are involved in the development of these 2 types of PCLBCLs. Further analysis identified 71 statistically significant differentially expressed genes as most discriminating genes between PCFCCLs and PCLBCLs-leg with fold changes of at least 3.0. Forty-eight genes were highly expressed in PCLBCLs-leg, and 23 genes showed high expression in PCFCCLs. The high expression of 5 of 6 selected genes in PCLBCLs-leg was confirmed by qPCR.
The increased cellular proliferative activity in PCLBCLs-leg, as shown by the high expression of various genes associated with proliferation as well as the high percentages Ki-67–positive staining tumor cells (> 75% in 12 of 13 cases), may probably be a result of the deregulated expression of several oncogenes with cell cycle regulatory functions such as CMYC, PIM1, and BCL6.29 In the group of PCLBCLs-leg high expression of CMYC was found, although the levels of gene expression values varied among individual samples. Interestingly, a correlation could be found between the highest CMYC expression value on microarray and qPCR (case no. 12) and the presence of a chromosomal translocation involving the IGH gene and CMYC gene (t(8;14)(q24;q32)) detected by fluorescence in situ hybridization (FISH) analysis performed in a separate study.30 In a small CGH-array study Mao et al17 showed gains of CMYC (8q24) in 2 of 4 PCLBCLs, but did not mention the localization of skin lesions in these cases. The oncogene CMYC is a transcription factor and has been proposed to be involved in multiple cellular functions such as cell cycle regulation, apoptosis, cell growth, metabolism, and differentiation, which might be cell type- and context-dependent.29 Overexpression of the CMYC gene by chromosomal translocation to the Ig loci is an important event in malignant transformation in virtually all Burkitt lymphomas, in 6% of DLBCLs, and recently found in 5 of 14 cases of PCLBCLs-leg.1,30,31 Interestingly, we also observed high expression of the PIM1 and PIM2 oncogenes in PCLBCLs-leg. Pim kinases are known to cooperate with CMYC and NMYC to generate T- and B-cell lymphomas, although the precise mechanism of this synergism in lymphomagenesis remains obscure.32-35 It is worth noting that a second transcriptional key regulator of cellular proliferation, Bcl-6, is expressed by the tumor cells of almost all PCLBCLs, and its deregulation may contribute to active cellular proliferation as well.10,14 Recently, somatic hypermutations of the BCL6 gene have been detected in a small series of PCLBCLs-leg, which may affect the Bcl-6–negative autoregulation circuit resulting in Bcl-6 deregulation as described in nodal diffuse large B-cell lymphoma.12,36,37
Another prominent distinction between gene expression profiles of PCLBCLs-leg and PCFCCLs was the marked high expression of B-cell transcription factors MUM1/IRF4 and OCT2 in PCLBCLs-leg, both expressed in late plasma cell–directed B-cell differentiation stages.38,39 Mum1/IRF4 is strongly expressed in lymphoplasmocytoid lymphoma, multiple myeloma, and 75% of diffuse large B-cell lymphomas.38 High MUM1/IRF4 expression in PCLBCLs-leg was confirmed by qPCR and immunohistochemistry and is consistent with the results of a recent study.12 MUM1/IRF4 is fused to the immunoglobulin locus in some cases of multiple myeloma and can function as an oncogene in vitro.40 MUM1/IRF4 is transiently induced during normal lymphocyte activation and is critical for the proliferation of B lymphocytes in response to signals from the antigen receptor.41,42 Thus, the constitutive expression of Mum1/IRF4 also may contribute to the unchecked proliferation of the malignant cells in PCLBCLs-leg. In addition, in vitro experiments demonstrated that expression of MUM1/IRF4 enhances the susceptibility of lymphocytes to undergo Fas-induced apoptosis.43 It has not yet been elucidated whether the Mum1/IRF4-mediated proapoptotic effects are selectively bypassed in Mum1/IRF4 expressing tumors, but it has been reported that Mum1/IRF4 function can be modulated by the presence of Bcl-6.44 Given the coexpression of Bcl-6 and Mum1/IRF4 in PCLBCLs-leg, it is possible that Bcl-6 may interfere with the proapoptotic effects of Mum1/IRF4.
In the group of PCFCCLs very high expression levels of the SPINK2 transcript were observed in all samples except one, whereas expression of SPINK2 was very low or absent in all samples of PCLBCLs-leg. Interestingly, SPINK2 was identified as the most differentially expressed gene between both groups, with a remarkable high fold change of 17.0. Furthermore, SPINK2 was recognized as one of the genes of the germinal center genes of the 43 genes that discriminate between the GCB and ABC types of DLBCLs with highest significance.28 The SPINK2 gene encodes a Kazal-type serine threonine kinase with ill-defined function in physiological and pathological cellular processes.45 Further studies will be needed to elucidate the potential role of SPINK2 in the development of PCFCCLs. In addition, several genes associated with the host immune response (granzyme M, selectin P, LCK) were substantially up-regulated in the group of PCFCCLs, which is consistent with the higher numbers of host immune cells in these tumors. This is also in line with previous observations in diffuse large B-cell lymphomas that genes associated with the host immune response and extracellular matrix components (both were considered part of the “lymph node signature”) are more highly expressed in GCB tumors.46
Consistent with previous studies,9,10 we observed strong Bcl-2 protein expression in 11 of 13 PCLBCLs-leg, but not in the PCFCCLs included for this study. BCL2 encoding mRNA expression values, however, did not statistically significantly differ between the 2 groups; BCL2 mRNA was detected in all patients both by using the microarray analysis (not discriminating between both splice variants) as well as qPCR analysis (for each individual splice variant). The presence of BCL2 mRNA despite the absence of Bcl-2 protein in the group of PCFCCLs was unexpected but has previously been described as a characteristic feature of germinal center B cells.47,48 It was suggested that germinal center cells may be involved in some arrest of Bcl-2 protein expression at the posttranscriptional level.49,50 Such a hitherto unknown mechanism might be conserved in PCFCCLs and reflect a typical feature of its proposed germinal center B-cell origin. The mechanisms underlying the high Bcl-2 protein expression in PCLBCLs-leg are at present unknown. Posttranslational modification such as ubiquitination of kinase sites or decreased degradation, for instance, by inhibition of FRAP/RAFT/mTOR, leading to increased cellular concentration of Bcl-2 protein unrelated to the amount of BCL2 mRNA might be a possible explanation.47,48
In conclusion, the results of the present study provide molecular support for the view that PCFCCLs and PCLBCLs-leg are distinct subtypes of PCLBCLs, as recognized in the EORTC classification. They also suggest different mechanisms of malignant transformation in these 2 types of PCLBCLs. The observation that PCFCCLs and PCLBCLs-leg have gene expression profiles similar to that of GCB and ABC type of diffuse large B-cell lymphoma is clinically important. It is not only consistent with the much better prognosis of the group of PCFCCLs but also suggests that different therapeutic approaches are warranted in these 2 types of PCLBCLs.
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-04-1594.
J.J.H. and R.D. contributed equally to this work.
Supported by grants from The Netherlands Organisation for Health Research and Development (907-00-066) (M.V.) and Stichting De Drie Lichten (J.H.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Professor R. Dalla-Favera for helpful discussions and critically reviewing the manuscript. We thank Enno Dreef and Aat Mulder for their excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal