Abstract
Autoimmune hemolytic anemia (AIHA) is the result of increased destruction of red blood cells (RBCs) due to the production of autoantibodies, and it can be life-threatening. To study the mechanisms that trigger AIHA, we used the Marshall-Clarke and Playfair model of murine AIHA, in which mice repeatedly immunized with rat RBCs develop erythrocyte autoantibodies as well as rat-specific alloantibodies. We analyzed the role of CD25+ T-regulatory subsets in controlling AIHA in C57/Bl6 mice using antibody depletion studies. Treatment with anti-CD25 antibody but not isotype control prior to immunization with rat RBCs increased the incidence of AIHA from 30% to 90%. Adoptive transfer of purified splenic population of CD4+CD25+ but not CD4+CD25- cells from immunized mice into naive recipients prevented the induction of autoantibody production. Altogether, our data establish a critical role for CD4+CD25+ cells for control of AIHA, which may help to establish therapeutic strategies for treatment of AIHA.
Introduction
Autoimmune hemolytic anemia (AIHA) is an autoimmune disease in which red blood cells (RBCs) are destroyed prematurely due to the production of antibodies against the patient's own red cells.1 The most common form of AIHA is characterized by the presence of “warm”-type autoantibodies, which are immunoglobulin G (IgG) type and react optimally at 37°C, causing RBC destruction extravascularly by tissue macrophages.1 Playfair and Marshall-Clarke described a mouse model of AIHA, in which repeated injections with rat RBCs result in the development of autoantibodies against self RBCs and a disease process similar to warm-type AIHA in humans with evidence of anemia, reticulocytosis, shortened survival of RBCs, and a direct Coombs-positive state.2 As expected, the mice develop rat-specific xenoantibodies, thus providing an inbuilt control for the autoantibody response.3,4 Spleen cells or T cells from mice immunized with rat RBCs transferred to naive recipients suppress the subsequent induction of autoantibodies, but not the xenoantibody response, indicating a regulatory mechanism in the induction of autoantibody response.4,5 The nature of this suppressive activity remains to be elucidated.
T-regulatory CD4+ cell populations characterized by coexpression of CD25 (interleukin-2 receptor alpha chain) have been shown to play an important role in the generation and maintenance of peripheral tolerance.6 Although studies have not directly addressed the role of CD4+CD25+ T cells in the development of AIHA per se, it was noted that about 25% of older CD25 knockout mice develop autoimmune disorders, including hemolytic anemia.7
We have used the Marshall-Clarke and Playfair model of murine AIHA to determine the role of CD4+CD25+ T-regulatory cells for induction of AIHA. Depletion studies using anti-CD25 increased the incidence of AIHA in C57/Bl6 mice from 30% to 90%. In addition, adoptive transfer of purified CD4+CD25+, but not CD4+CD25- cells, from immunized mice prevented the induction of AIHA. Altogether, our data demonstrate the importance of CD4+CD25+ cells for control and induction of AIHA.
Study design
Immunization regimen for induction of AIHA
Rat RBCs were isolated from whole rat blood using histopaque (Sigma-Aldrich, St. Louis, MO) gradient and adjusted to 109 cell/mL. Female C57/Bl6 mice between 8 and 10 weeks old were immunized intraperitoneally with 2 × 108 rat RBCs in 200 μL RPMI on a weekly basis.
In vivo depletion with anti-CD25
C57/BL6 mice were given 500 μg anti-CD25 (clone 7D4; BD Pharmingen, San Diego, CA) on a weekly basis for a total of 3 weeks, 8 hours prior to immunization with rat RBCs. As control, mice were treated with 500 μg isotype control rat IgG2b (BD Pharmingen) followed by immunization with rat RBCs or with 500 μg anti-CD25 alone without the rat RBC immunization regimen.
Detection and measurement of auto- and alloantibodies and complete hematologic analysis
Blood samples (25 μL) by retro-orbital sinus bleeding were obtained on a weekly basis, 5 days after each immunization. Reticulocyte counts were performed using the Advia 120 Hematology System (Bayer, Tarrytown, NY). In addition, levels of IgG sensitization (autoantibodies) on the RBCs were determined by flow cytometry using fluorescein isothiocyanate (FITC)–conjugated anti–mouse IgG (Vector Laboratories, Burlingame, CA). For analysis of rat RBC-specific xenoantibodies, rat RBCs were incubated with diluted mouse plasma for 1 hour at 37°C and after several washes, were stained with FITC-conjugated anti–mouse IgG. Analysis of levels of anti–double stranded (ds) DNA was performed with plasma diluted at a final concentration of 1 in 100 using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (Alpha Diagnostic, San Antonio, TX).
Transfusion studies
Mouse RBCs obtained from naive female C57/Bl6 mice were labeled with PKH-26 (Sigma)8 and injected by the tail-vein into control mice or those that had developed AIHA. Blood samples were obtained by retro-orbital sinus bleeding at the time points indicated after transfusion and the clearance of fluorescent RBCs was measured by flow cytometry as previously described.8
Adoptive cell transfer
Splenocytes from animals immunized with rat RBCs on a weekly basis for 12 weeks were harvested, stained with phycoerythrin (PE)–labeled anti-CD4 (clone) and PE-Cy7–labeled anti-CD25 (clone 7D4) (both from BD Pharmingen) and purified using high-speed sorting (MoFlo; Dako Cytomation, Carpinteria, CA) to separate CD4+CD25- and CD4+CD25hi cells. Approximately 2 × 105 cells in 0.2 mL of phosphate-buffered saline (PBS) was injected intravenously into 10-week-old female C57/Bl6 recipient mice. After one day, the mice were immunized with rat RBCs on a weekly basis.
Statistical analysis
The significance of differences between groups of mice were calculated using a single-factor analysis of variance (Anova) test, and only P values below .05 were considered significant.
Results and discussion
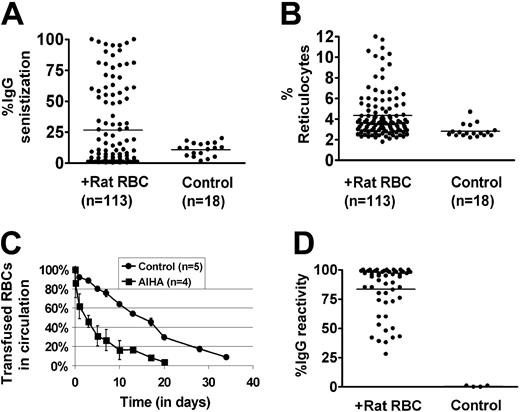
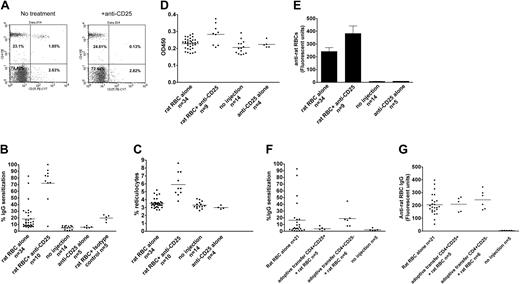
Approximately 30% of female C57/Bl6 mice immunized on a weekly basis with rat RBCs develop AIHA, as evidenced by presence of red cell–specific autoantibodies on their RBCs, increased levels of reticulocytes, and increased destruction of transfused syngeneic mouse RBCs (Figure 1A-C). However, all the mice developed rat RBC-specific xenoantibodies (Figure 1D), consistent with previous data.4 To examine whether CD4+CD25+ cells play a role in the induction of AIHA in this experimental model, we depleted mice (n = 10) of CD25+ cells prior to immunization with rat RBCs on a weekly basis for 3 weeks. Administration of an anti-CD25 antibody almost completely depleted CD4+CD25+ cells in C57/Bl6 mice (Figure 2A). Immunization of rat RBCs in the depleted mice increased the frequency of AIHA to 90% (Figure 2B-C), demonstrating a critical role for T-regulatory CD25+ cells in development of this type of autoimmune disease. Weekly treatment with an isotype-control antibody followed by rat RBC immunization (n = 5) (Figure 2B) or treatment with anti-CD25 alone (n = 5) (Figure 2B-C) for 3 weeks did not result in increased incidence of AIHA as observed in the anti-CD25/rat RBC–immunized mice.
Development of AIHA in mice treated on a weekly basis with rat RBCs. Female C57/Bl6 mice age 8 to 10 weeks were immunized on a weekly basis with rat RBCs for 10 weeks. (A) Levels of IgG-specific autoantibodies on mouse RBCs were measured by flow cytometry and are expressed as percentage of background unstained cells. The bars in each panel represent the mean values and n is the number of mice in each group. The autoantibody levels in rat immunized compared with control uninjected mice were significantly higher (P < .05). (B) Numbers of circulating reticulocytes in mice expressed as a percentage. There was a significant difference in the reticulocyte counts between the rat-immunized group and control uninjected mice (P < .05). (C) RBCs obtained from mice immunized with rat RBCs (n = 4) or control mice (n = 5) were fluorescently labeled with PKH-26 and injected intravenously into an equivalent number of C57Bl/6-naive mice. At times indicated, venous blood was sampled and analyzed by flow cytometry for the fraction of fluorescent RBCs. To show the clearance kinetics, injected RBCs at 1 minute after injection were taken as 100%, and the remaining RBCs were calculated at different time points as the average for each group of mice (error bars depict the standard error of the mean SEM). The difference at all points between the mice receiving RBCs from rat-immunized mice and those receiving control RBCs was calculated as P = .04. (D) Levels of IgG-specific anti–rat xenoantibodies in plasma were measured by first incubating rat erythrocytes with diluted mouse plasma (1 in 50 000) followed by staining with FITC-conjugated anti–mouse IgG. The analysis was performed by flow cytometry and is presented as relative fluorescent units on the y axis.
Development of AIHA in mice treated on a weekly basis with rat RBCs. Female C57/Bl6 mice age 8 to 10 weeks were immunized on a weekly basis with rat RBCs for 10 weeks. (A) Levels of IgG-specific autoantibodies on mouse RBCs were measured by flow cytometry and are expressed as percentage of background unstained cells. The bars in each panel represent the mean values and n is the number of mice in each group. The autoantibody levels in rat immunized compared with control uninjected mice were significantly higher (P < .05). (B) Numbers of circulating reticulocytes in mice expressed as a percentage. There was a significant difference in the reticulocyte counts between the rat-immunized group and control uninjected mice (P < .05). (C) RBCs obtained from mice immunized with rat RBCs (n = 4) or control mice (n = 5) were fluorescently labeled with PKH-26 and injected intravenously into an equivalent number of C57Bl/6-naive mice. At times indicated, venous blood was sampled and analyzed by flow cytometry for the fraction of fluorescent RBCs. To show the clearance kinetics, injected RBCs at 1 minute after injection were taken as 100%, and the remaining RBCs were calculated at different time points as the average for each group of mice (error bars depict the standard error of the mean SEM). The difference at all points between the mice receiving RBCs from rat-immunized mice and those receiving control RBCs was calculated as P = .04. (D) Levels of IgG-specific anti–rat xenoantibodies in plasma were measured by first incubating rat erythrocytes with diluted mouse plasma (1 in 50 000) followed by staining with FITC-conjugated anti–mouse IgG. The analysis was performed by flow cytometry and is presented as relative fluorescent units on the y axis.
Antibody depletion and adoptive transfer studies to study the role of CD4+CD25+ T cells in induction of AIHA. (A) A representative flow cytometric analysis of peripheral blood leukocytes obtained from mice before (left) and 4 days after (right) intraperitoneal injection of 500 μg monoclonal anti-CD25 (clone 7D4). Cells were stained with PE-conjugated monoclonal anti-CD4 (GK1.5) and PE-Cy7–conjugated anti-CD25 (clone PC61.5). (B) Levels of IgG-specific autoantibodies on RBCs from mice expressed as percentage of background unstained cells. The difference between rat RBC/anti-CD25 and rat RBC-alone groups was calculated as P < .001, and between rat RBC/anti-CD25 and rat RBC/isotype control groups as P < .001. As expected, the rat RBC/isotype-control group was different from the no-injection control group (P < .001), but no differences were found between the rat RBC/isotype-control and rat RBC-alone groups (P = .9). In addition, there were no differences between no-injection and anti-CD25 alone–injected groups (P = .5). (C) The circulating reticulocyte counts of the mice are expressed as a percentage of total blood. The difference between rat RBC/anti-CD25 and rat RBC-alone groups was P = 4 × 10-9. No differences were found between the no-injection and anti-CD25 alone groups (P = .2). (D) The relative levels of anti–ds DNA in plasma of mice were measured by ELISA using a commercially available kit and are presented in optical density (OD) units on the y-axis. The difference between rat RBC/anti-CD25 and rat RBC-alone groups was calualted as P = .001. (E) The presence of IgG-specific xenoantibodies to rat RBCs in plasma from mice at 3 weeks after immunization was measured using diluted plasma (1 in 1000) followed by analysis using flow cytometry and is presented as relative fluorescent units on the y-axis. The difference between rat RBC/anti-CD25 and rat RBC-alone groups was calculated as P = .04. Error bars indicate SEM. (F) About 2 × 105 CD4+CD25hi or CD4+CD25- sorted cells were adoptively transferred into naive C57Bl/6 female mice followed by weekly immunization with rat RBCs for 9 weeks. Levels of IgG autoantibodies on mouse RBCs as well as (G) xenoantibodies (IgG) to rat RBCs are shown for individual mice. The difference in autoantibody levels between mice adoptively transferred with CD4+CD25hi and those that received CD4+CD25- was calculated as P = .04. However, there were no differences in the xenoantibody levels between these 2 groups (P = .4). In panels B-D and F-G, the horizontal lines indicate the mean values.
Antibody depletion and adoptive transfer studies to study the role of CD4+CD25+ T cells in induction of AIHA. (A) A representative flow cytometric analysis of peripheral blood leukocytes obtained from mice before (left) and 4 days after (right) intraperitoneal injection of 500 μg monoclonal anti-CD25 (clone 7D4). Cells were stained with PE-conjugated monoclonal anti-CD4 (GK1.5) and PE-Cy7–conjugated anti-CD25 (clone PC61.5). (B) Levels of IgG-specific autoantibodies on RBCs from mice expressed as percentage of background unstained cells. The difference between rat RBC/anti-CD25 and rat RBC-alone groups was calculated as P < .001, and between rat RBC/anti-CD25 and rat RBC/isotype control groups as P < .001. As expected, the rat RBC/isotype-control group was different from the no-injection control group (P < .001), but no differences were found between the rat RBC/isotype-control and rat RBC-alone groups (P = .9). In addition, there were no differences between no-injection and anti-CD25 alone–injected groups (P = .5). (C) The circulating reticulocyte counts of the mice are expressed as a percentage of total blood. The difference between rat RBC/anti-CD25 and rat RBC-alone groups was P = 4 × 10-9. No differences were found between the no-injection and anti-CD25 alone groups (P = .2). (D) The relative levels of anti–ds DNA in plasma of mice were measured by ELISA using a commercially available kit and are presented in optical density (OD) units on the y-axis. The difference between rat RBC/anti-CD25 and rat RBC-alone groups was calualted as P = .001. (E) The presence of IgG-specific xenoantibodies to rat RBCs in plasma from mice at 3 weeks after immunization was measured using diluted plasma (1 in 1000) followed by analysis using flow cytometry and is presented as relative fluorescent units on the y-axis. The difference between rat RBC/anti-CD25 and rat RBC-alone groups was calculated as P = .04. Error bars indicate SEM. (F) About 2 × 105 CD4+CD25hi or CD4+CD25- sorted cells were adoptively transferred into naive C57Bl/6 female mice followed by weekly immunization with rat RBCs for 9 weeks. Levels of IgG autoantibodies on mouse RBCs as well as (G) xenoantibodies (IgG) to rat RBCs are shown for individual mice. The difference in autoantibody levels between mice adoptively transferred with CD4+CD25hi and those that received CD4+CD25- was calculated as P = .04. However, there were no differences in the xenoantibody levels between these 2 groups (P = .4). In panels B-D and F-G, the horizontal lines indicate the mean values.
We also measured the levels of anti–ds DNA characteristic of systemic autoimmune disease and found significantly elevated levels in anti-CD25/rat RBC–immunized mice, as compared with control mice treated with only rat RBCs or treated with anti-CD25 alone (Figure 2D). In addition, the levels of xenoantibodies against rat RBCs in anti-CD25/rat RBC–immunized mice were elevated as compared with mice treated with rat RBCs alone (Figure 2E), consistent with a heightened immune hypersensitive state.
Splenocytes taken from mice immunized with rat RBCs at weekly intervals for 12 weeks and transferred to naive recipients suppress the subsequent induction of autoantibodies.4 To determine if CD4+CD25+ cells play a role in this suppressive activity in this animal model, we adoptively transferred CD4+CD25+ or CD4+CD25- sorted cells from mice immunized with rat RBCs on a weekly basis for 12 weeks, into naive C57/Bl6 mice. One day after the transfer, mice were immunized with rat RBCs on a weekly basis. Analysis of the mice after 9 immunizations demonstrated that autoantibody production was completely suppressed in mice adoptively transferred with CD4+CD25+ (n = 5), but not those transferred with CD4+CD25- (n = 6) cells (Figure 2F). Analysis of anti–rat RBC levels indicated that CD4+CD25+ adoptively transferred mice developed xenoantibodies as effectively as control mice (Figure 2G), demonstrating that CD4+CD25+ suppressive activity is autoantibody-response specific.
Although there may be multiple factors that contribute to the induction of AIHA, our data indicate that defective suppressive activity of CD4+CD25+ cells could play an essential role in the onset and/or maintenance of this autoimmune disease. Current therapies for AIHA include administration of immunosuppressive drugs together with transfusion or splenectomy in patients with relapsing cases, therapies that typically control, rather than cure, the disease.1 The immunotherapeutic potential of CD4+CD25+ cells may represent a more effective strategy for treatment of AIHA.
Prepublished online as Blood First Edition Paper, January 6, 2005; DOI 10.1182/blood-2004-12-4692.
Supported in part by grants from the National Institutes of Health (R01 HL69 102) and the American Heart Association Grant-in-Aid Heritage Affiliate.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Yazan Abdullah for his technical assistance in setting up the immunization protocol and performing the initial measurements of the auto- and xenoantibodies. We are grateful to GabrielAlespeti (New York Blood Center) for cell sorting and advice on some of the FACS analysis. We wish to thank Dr Barry Coller (Rockefeller University) for allowing us to use hisAdvia 120 hematology system and are especially grateful to Thomas Hoffman for his help with the instrument.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal