The life cycle of dendritic cells (DCs) must be precisely regulated for proper functioning of adaptive immunity. However, signaling pathways actively mediating DC death remain enigmatic. Here we describe a novel mechanism of hierarchical transcriptional control of DC life and death. Ligation of tumor necrosis factor receptor superfamily (TNFR-SF) members on DCs and cognate contact with T cells resulted in quantitatively balanced nuclear factor-κB (NF-κB) and c-Jun N-terminal kinase (JNK)–mediated activator protein-1 (AP-1) induction and strongly enhanced DC longevity. Specific blockade of NF-κB in DCs induced strongly augmented JNK/AP-1 activity because of elevated levels of reactive oxygen species. In this scenario, DC activation by TNFR-SF members or T cells induced DC apoptosis. Specific inhibition of JNK/AP-1 rescued DCs from this activation-induced cell death program and restored TNFR-SF member- and T-cell–mediated survival. We conclude that JNK/AP-1 activity is under negative feedback control of NF-κB and can execute apoptosis in DCs. Thus, feedback-controlled signaling amplitudes of 2 transcriptional pathways decide the fate of a DC.
Introduction
Elimination of dendritic cells (DCs) in experimental animals leads to immunologic ignorance or even paralysis of antigen-specific T cells after antigen exposure.1 Accordingly, the induction of premature DC death by immunomodulatory drugs appears to be a major pharmacologic principle of anti-inflammatory treatments.2 In contrast, the prolongation of DC survival by caspase mutations is associated with the occurrence of lymphoproliferative disease and autoimmunity in humans.3 Autoimmunity is also induced when DCs are chronically stimulated through the antiapoptotic effector molecule CD40.4 Thus, DC survival and death induction must be tightly regulated to ensure the proper functioning of adaptive immunity.
DC survival appears to be actively regulated during communication between DCs and other cells of the immune system. Antiapoptotic receptors of DCs include CD405 and receptor activator of nuclear factor-κB (RANK).6,7 The ligands of these receptors (CD40L and RANKL) are mainly expressed by activated T cells. DC–T-cell communication can also result in DC death.8 However, surface receptors that induce apoptosis in other cells (eg, CD95/Fas) surprisingly can trigger activation rather than death in DCs.9 Thus, the display of proapoptotic and antiapoptotic receptors by DCs does not allow a reliable prediction of the biologic consequences after their ligation. Therefore, either the relevant proapoptotic receptors of DCs are yet not defined or the critical decisions regarding DC fate are made by tuning the signaling cascades of established receptors.
Various pharmacologic studies have suggested essential contributions of nuclear factor-κB (NF-κB), extracellular signal–regulated kinase 1/2 (ERK 1/2), and phosphatidylinositol-3 kinase signaling pathways to DC survival.10-12 Recent data indicate that DCs of NF-κB family member p50 and cRel double-knockout (KO) mice are refractory to the survival-promoting effects of CD40L and RANKL.13 Thus, some DC survival–promoting signaling pathways are at least partly understood. However, pathways that critically regulate cell death in other cells, such as c-Jun N-terminal kinase/activator protein-1 (JNK/AP-1) signaling have not been studied in DCs in this respect. Hence, we still lack knowledge of the signaling events that actively execute DC death programs.
In this study, we used human CD34+ hematopoietic progenitor cell (HPC)–derived DCs and human skin DCs isolated ex vivo to identify transcriptional programs linked to the survival or the death of DCs during T-cell encounter and on the triggering of defined surface receptors. Our data show that the type of signaling pathway activated, rather than the type of surface receptor engaged, decides whether a DC is rescued from apoptosis or undergoes activation-induced cell death.
Materials and methods
Antibodies and reagents
Nonconjugated monoclonal antibodies (mAbs) included anti–HLA-DR (MEM-136; provided by Dr V. Horejsi, Academy of Natural Sciences, Prague, Czech Republic) and anti-CD40L (clone 24-31; Ancell, Bayport, MN). Goat F(ab')2 anti–mouse immunoglobulin G (IgG) conjugated to tetramethylrhodamine-5-(and 6)-isothiocyanate (TRITC) was from Jackson ImmunoResearch Laboratories (West Grove, PA), and 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) was from Molecular Probes (Eugene, OR). Cycloheximide was from Sigma (St Louis, MO). JNK-specific inhibitor SP600125, p38 mitogen-activated protein kinase (MAPK) inhibitor PD169316, and MAP/ERK kinase (MEK) inhibitor U0126 were from Calbiochem (Bierges, Belgium). Caspase-3 (Z-DEVD-FMK) and caspase-8 (Z-IETD-FMK)–specific inhibitors were from Alexis Biochemicals (Bayport, MN).
Growth factors and cytokines
Human recombinant granulocyte macrophage–colony-stimulating factor (rGM-CSF) was obtained from Novartis (Basel, Switzerland). Tumor necrosis factor-α (TNF-α) and fms-like tyrosine kinase 3 ligand (Flt3L) were from Strathmann Biotech AG (Hannover, Germany). Purified human transforming growth factor-β1 (TGF-β1) was from R&D Systems (Minneapolis, MN). FLAG-tagged CD40L, CD95L, and TNF-related apoptosis-inducing ligand (TRAIL) were from Alexis Biochemicals. FLAG-tagged molecules were used with anti-FLAG M2 mAb (Sigma; 10 μg/mL each).
T-cell preparations
CD8+ and naive CD4+ T cells were obtained from adult peripheral blood mononuclear cells (MNCs), as described,14 with the modification of adding anti-CD11b and anti-CD34 (both from Immunotech, Marseille, France) to the mAb depletion cocktails. T-cell populations were more than 95% pure, as determined by FACS analysis (BD Biosciences, Franklin Lakes, NJ).
Generation of Langerhans cell–type DCs from CD34+ HPCs
CD34+ HPCs were separated from cord blood MNCs by positive immunoselection (Direct CD34+ Progenitor Cell Isolation Kit; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). CD4+ and CD8+ cord blood T cells were purified as described.15 CD34+ HPCs were cultured in RPMI 1640/10% fetal calf serum (FCS) supplemented with GM-CSF (200 U/mL)/TNF-α (50 U/mL)/Flt3L (50 ng/mL) as described.16 On day 6 (d6), medium was supplemented with TGF-β1 (0.1 ng/mL) to generate LC–type DCs.17 On d10, cells were harvested and cultured in fresh medium supplemented with GM-CSF/TNF-α until d11.5.
Preparation of skin Langerhans cells
Split-thickness skin (0.8 mm) was obtained on informed consent from healthy adults undergoing elective surgery (breast reduction and abdominoplasty). Epidermal sheets were prepared after incubation of split-thickness skin with dispase (50 U/mL; Invitrogen, San Diego, CA) for 60 minutes at 37°C, as described,18 and single cells were released by incubation in trypsin/EDTA (ethylenediaminetetraacetic acid) (Invitrogen) for 30 minutes at 37°C and vigorous agitation. Cell suspensions were sedimented over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) at 350g for 30 minutes. Interphase cells used for transfection contained between 10% and 20% anti–CD1a-reactive LCs.
RT-PCR cloning
cDNAs were amplified by reverse transcription–polymerase chain reaction (RT-PCR) using the primers described in the supplemental information (see the Supplemental Data link at the top of the online article on the Blood website). Specific amplicons were inserted into a pcDNA3.1 eukaryotic expression vector (Invitrogen) and sequenced.
Plasmids
DNA encoding inhibitor of NF-κBα (IκBα carrying mutations converting serine residues at positions 32 and 36 into alanin (IκBαAA) in pUSE-amp(+) was obtained from UpstateGroup (Charlottesville, VA). The coding sequence was subcloned into p-internal ribosome entry site 2–enhanced green fluorescence protein (pIRES2-EGFP) (BD Biosciences) to give IκBαAA-pIRES2-EGFP. Secreted human placental alkaline phosphatase (hPAP)–encoding reporter plasmids were obtained from BD Biosciences to survey the specific cis-acting induction of cyclic adenosine monophosphate (cAMP) response element (CRE) and serum response element (SRE) or the enhancer elements activated by AP-1, NF-κB, or nuclear factor of activated T cell (NFAT). A modified thymidine kinase (TK) promoter upstream of hPAP (pTA-secreted alkaline phosphatase, pTA-SEAP) and promoterless plasmids (both from BD Biosciences) were used as controls. Bcl-2 cDNA in a pcDNA3.1 expression vector was a gift from Dr C. Borner (University of Freiburg, Germany). Plasmids encoding wild-type JNK1 (WT-JNK1) and dominant-negative JNK1 (DN-JNK1) were obtained from Dr S.-I. Hirai (University School of Medicine, Yokohama, Japan).19 For transfections, plasmids were isolated using EndoFree Plasmid Kit (Qiagen, Valencia, CA).
Transfection of DCs
DCs were transfected as described previously.15 Briefly, 1 to 2 × 107 d11.5 HPC-derived DCs or interphase cells obtained from epidermal cell suspensions were electroporated with 20 μg plasmid DNA in 800 μL transfection buffer (21 mM HEPES [N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid], 137 mM NaCl, 0.7 mM Na2HPO4, 5 mM KCl, 6 mM glucose) at 960 μF/390 V in 0.8-cm gap-size cuvettes on a GenePulser (both from Bio-Rad Laboratories, Hercules, CA). After transfection, HPC-derived DCs were plated in culture supernatant from DCs harvested before transfection. Transfected skin LCs were plated in RPMI 1640/10% FCS supplemented with GM-CSF (200 U/mL). For functional assays, cells were harvested 12 hours after transfection and were stained with CyChrome-conjugated anti-CD1a mAb (HI 149; BD Biosciences), as described.16 HPC-derived EGFP+/CD1a+ DCs were subjected to fluorescence-activated cell sorting (FACS) (FACStarplus; BD Biosciences) and were used for further experiments. The purity of sorted cell populations was regularly greater than 98%.
Western blotting and phosphoprotein assays
Cellular extracts were subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred onto nitrocellulose membranes (Bio-Rad Laboratories). Membranes were incubated with mouse anti–c-Jun (J31920; BD Biosciences), phosphorylation site-specific mouse anti–c-Jun (Ser63, clone KM-1; Santa Cruz Biotechnology, Santa Cruz, CA), or rabbit anti-IκBα (C-21; Santa Cruz), and the binding of primary antibodies was revealed as described.20 Membranes were stripped and reprobed with rabbit anti–actin antibodies (A2066; Sigma) to control for equal loading and transfer of proteins. Alternatively, cells were fixed and permeabilized (Fix&Perm; An der Grub, Kaumberg, Austria) and were exposed to phosphorylation site-specific anti-JNK (Thr183/Tyr185, clone G9; Cell Signaling Technology, Beverly, MA) and anti–c-Jun mAbs or isotype-matched control mAbs (Sigma). Binding of mAbs was revealed by phycoerythrin (PE)–conjugated goat F(ab')2 anti–mouse IgG (Molecular Probes), and PE fluorescence of EGFP+ DCs was recorded on a FACScan flow cytometer (BD Biosciences).
T-cell–DC cocultures
FACS-sorted DCs (5 × 103) were seeded into 96-well flat-bottom plates in RPMI 1640/10% FCS. Where indicated, DCs were pulsed for 30 minutes with 1 μg/mL TSST-1 (Toxin Technology, Beverly, MA) and washed. Finally, 3 to 5 × 105 purified T cells or the indicated concentrations of recombinant ligands were added per well, and cultures were grown for the indicated time periods.
Determination of DC viability
To assess the survival of FACS-sorted CD1a+/CFSE+ (nontransfected) or CD1a+/EGFP+ (transfected) HPC-derived DCs and CD1a+/EGFP+ epidermal LCs, cells were seeded at a density of 1 to 5 × 103 cells/mL into 96-well flat-bottom plates in the absence or presence of T cells or the indicated recombinant ligands. Cells were harvested, and cell clusters were disrupted by pipetting in phosphate-buffered saline (PBS)/10 mM EDTA. Cells were washed twice in ice-cold Annexin V binding buffer, labeled with Annexin V-PE (both from Bender MedSystems, Vienna, Austria) and anti–CD3-peridinin chlorophyll protein (PerCP; BD Biosciences), and analyzed on a FACScan. Viable transfected DCs retained strong EGFP fluorescence for more than 5 days after transfection (data not shown).
Reporter gene assays
DCs were transfected with the respective reporter constructs and placed in culture, and cell-free supernatants were collected 36 to 48 hours later. Reporter products in supernatants were quantified as specified in the supplemental information.
RNA expression profiling
Total RNA was subjected to 2 rounds of linear amplification.21 Biotin-labeled ribonucleotides were incorporated using the ENZO Bio-Array High-Yield RNA Transcript Labeling Kit (both from Affymetrix, Santa Clara, CA) during the second round of in vitro transcription. Ten micrograms fragmented cRNA was hybridized to Human Genome U133 Plus 2.0 Array (Affymetrix). Data analysis was performed using Microarray Suite software version 5.0 (MAS5.0; Affymetrix).
Quantitative PCR
Quantitative PCR (qPCR) was performed according to standard procedures, as described in the supplemental information.
Detection of carbonylated proteins
Carbonylated proteins were revealed using the OxyBlot Protein Oxidation Detection Kit (Chemicon, Temecula, CA) according to the instructions of the manufacturer. Briefly, cells were lysed in 6% SDS, and 2,4-DNP-hydrazine was added to derivatize the carbonyl groups in the protein side chains to 2,4-DNP-hydrazone. After 15 minutes, the reaction was stopped, and proteins were separated by SDS-PAGE; this was followed by Western blot analysis and the detection of DNP-derivatized proteins by anti–DNP antibodies.
Results
DC fate is determined by cognate T-cell interaction and CD40 triggering
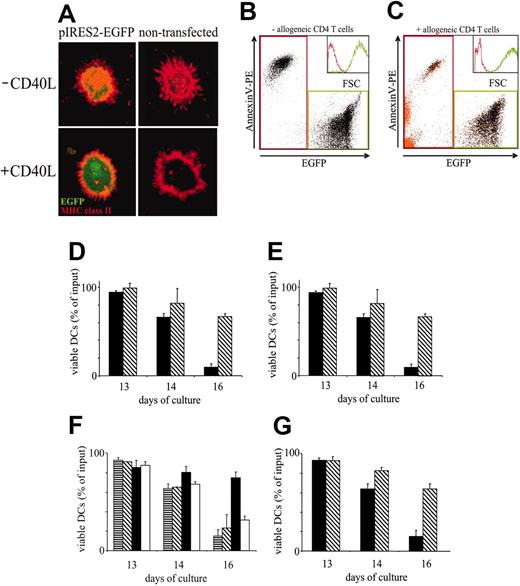
We established an experimental setup to analyze the effect of cognate DC–T-cell contact and of surface receptor ligation by recombinant ligands on the longevity of transfected and native DCs. DCs were differentiated from purified CD34+ HPCs and were transfected at d11.5 of their development with vectors encoding EGFP. DCs were sorted according to green fluorescence and anti-CD1a immunoreactivity 16 hours after transfection. Successfully transfected DCs, as control DCs, displayed major histocompatibility complex class II (MHC II) molecules in cytoplasmic organelles and redistributed MHC II to the surface membrane after treatment with soluble CD40L (Figure 1A). Other CD40L-induced DC functions (ie, allostimulation, induction of Th1 cells and CTLs, migration to CC chemokine receptor 7 ligands) were similarly unaltered by transfection.15 To study survival, EGFP-expressing transfected and, for control purposes, fluorescein-labeled nontransfected DCs were cultured in the presence or absence of allogeneic CD4+ T cells until d16. As shown in Figure 1B-C, dead DCs could be distinguished from viable DCs by their lower forward light scatter (FSC) and EGFP expression intensity and their high Annexin V binding. These apoptosis-associated characteristics were equally observed in DCs dying in the absence (Figure 1B) and in the presence of allogeneic T cells (Figure 1C). The rate of DC apoptosis was, however, drastically reduced in the presence of T cells (compare the abundance of Annexin V+ black dots in Figure 1B-C). In the absence of T cells, more than 90% of transfected and of nontransfected DCs were lost until d16 (Figure 1D-E). In the presence of T cells, more than 60% of transfected and of nontransfected DCs were still viable at d16 (Figure 1D-E). Thus, T cells positively regulate DC survival, and marker gene transfection into DCs does not alter the ability of DCs to respond to survival signals induced by T cells.
DCs require CD4+ T cell–derived stimuli to escape apoptosis. (A-C) Assessment of DC survival. (A) Unperturbed distribution and regulation of MHC class II in transfected DCs. pIRES2-EGFP–transfected (left column) and nontransfected (right column) DCs were cultured in the absence (top row) or presence (bottom row) of CD40L. DCs immobilized onto adhesion slides were fixed, permeabilized, and stained with anti–HLA-DRβ mAbs followed by TRITC-labeled secondary reagent. Fluorescence was analyzed by laser scanning microscopy, as described.18 (B) Discrimination of viable and apoptotic DCs. DCs were transfected with pIRES2-EGFP and FACS sorted to a purity of 98%. EGFP+ DCs were cultured for 3 days after transfection, stained with annexin V–PE, and analyzed using FACS. Two major DC populations can be distinguished on the basis of EGFP (x-axis) and annexin V–PE binding (y-axis). EGFPlow/annexin V–PE+ cells correspond to dead/apoptotic DCs, and EGFPhigh/annexin V–PE– cells correspond to viable DCs. Dead (red gate) and viable (green gate) DCs are also clearly distinguished by their nonoverlapping FSC histograms (transparent inset: dead DCs, red line; viable DCs, green line). (C) Enumeration of viable DCs in DC–T-cell cocultures. FACS-sorted pIRES2-EGFP–transfected DCs and purified allogeneic CD4+ T cells were cocultured for 3 days. Cell conjugates were dissociated, and cells were stained with annexin V–PE and anti–CD3–PerCP and analyzed by FACS. CD3+ T cells were gated and are shown as orange dots. Four cell populations are resolved: EGFP–/annexin V– cells (viable T cells), EGFP–/annexin V+ cells (dead T cells), EGFPlow/annexin V-PE+ cells (dead DCs), and EGFPhigh/annexin V–PE– cells (viable DCs). Again, viable DCs (green gate) can be distinguished from dead T cells and dead DCs (red gate) by nonoverlapping FSC histograms, as shown in the transparent inset (green and red lines, respectively). Fewer than 3% of DCs remained conjugated to T cells (orange dots in green gate). (D-E) Comparable basal and T cell–dependent survival of nontransfected DCs (D) and EGFP-transfected sorted DCs (E). DCs were cultured in the absence (▪) or presence (▧) of allogeneic CD4+ T cells at a DC/T-cell ratio of 1:100 until day 16. Cells were harvested at the indicated time points, and numbers of viable DCs were determined by FACS analysis. (F) T-cell–dependent DC survival requires cognate DC–T-cell interaction. DCs were pulsed with 1 μg/mL TSST1 sAg or were left untreated. sAg-loaded or nonpulsed DCs were cultured alone or cocultured with autologous CD4+ T cells with or without 10 μg/mL anti-CD40L mAbs. ▥ indicates medium; ▧, autologous CD4+ T cells alone; ▪, with sAg; and □, with sAg and anti-CD40L mAbs. (G) DCs were cultured in the absence (▪) or presence (▧) of CD40L. (D-G) Results are presented as percentage of viable DCs after culture relative to the number of input DCs (mean ± SD of triplicate determinations). Results are representative of at least 3 independent experiments.
DCs require CD4+ T cell–derived stimuli to escape apoptosis. (A-C) Assessment of DC survival. (A) Unperturbed distribution and regulation of MHC class II in transfected DCs. pIRES2-EGFP–transfected (left column) and nontransfected (right column) DCs were cultured in the absence (top row) or presence (bottom row) of CD40L. DCs immobilized onto adhesion slides were fixed, permeabilized, and stained with anti–HLA-DRβ mAbs followed by TRITC-labeled secondary reagent. Fluorescence was analyzed by laser scanning microscopy, as described.18 (B) Discrimination of viable and apoptotic DCs. DCs were transfected with pIRES2-EGFP and FACS sorted to a purity of 98%. EGFP+ DCs were cultured for 3 days after transfection, stained with annexin V–PE, and analyzed using FACS. Two major DC populations can be distinguished on the basis of EGFP (x-axis) and annexin V–PE binding (y-axis). EGFPlow/annexin V–PE+ cells correspond to dead/apoptotic DCs, and EGFPhigh/annexin V–PE– cells correspond to viable DCs. Dead (red gate) and viable (green gate) DCs are also clearly distinguished by their nonoverlapping FSC histograms (transparent inset: dead DCs, red line; viable DCs, green line). (C) Enumeration of viable DCs in DC–T-cell cocultures. FACS-sorted pIRES2-EGFP–transfected DCs and purified allogeneic CD4+ T cells were cocultured for 3 days. Cell conjugates were dissociated, and cells were stained with annexin V–PE and anti–CD3–PerCP and analyzed by FACS. CD3+ T cells were gated and are shown as orange dots. Four cell populations are resolved: EGFP–/annexin V– cells (viable T cells), EGFP–/annexin V+ cells (dead T cells), EGFPlow/annexin V-PE+ cells (dead DCs), and EGFPhigh/annexin V–PE– cells (viable DCs). Again, viable DCs (green gate) can be distinguished from dead T cells and dead DCs (red gate) by nonoverlapping FSC histograms, as shown in the transparent inset (green and red lines, respectively). Fewer than 3% of DCs remained conjugated to T cells (orange dots in green gate). (D-E) Comparable basal and T cell–dependent survival of nontransfected DCs (D) and EGFP-transfected sorted DCs (E). DCs were cultured in the absence (▪) or presence (▧) of allogeneic CD4+ T cells at a DC/T-cell ratio of 1:100 until day 16. Cells were harvested at the indicated time points, and numbers of viable DCs were determined by FACS analysis. (F) T-cell–dependent DC survival requires cognate DC–T-cell interaction. DCs were pulsed with 1 μg/mL TSST1 sAg or were left untreated. sAg-loaded or nonpulsed DCs were cultured alone or cocultured with autologous CD4+ T cells with or without 10 μg/mL anti-CD40L mAbs. ▥ indicates medium; ▧, autologous CD4+ T cells alone; ▪, with sAg; and □, with sAg and anti-CD40L mAbs. (G) DCs were cultured in the absence (▪) or presence (▧) of CD40L. (D-G) Results are presented as percentage of viable DCs after culture relative to the number of input DCs (mean ± SD of triplicate determinations). Results are representative of at least 3 independent experiments.
We next asked whether T-cell–mediated DC survival requires cognate (ie, MHC II–dependent) T-cell activation. Sorted EGFP+ DCs were pulsed with toxic shock syndrome toxin-1 (TSST-1) superantigen (sAg) or were nonpulsed and cocultured with autologous CD4+ T cells. In the presence of T cells, 80% of TSST-1–pulsed DCs survived until d16 of culture (Figure 1F). In contrast, autologous T cells could not augment the survival of non–TSST-1–modified DCs. Similarly, sAg pulsing of DCs and culture in the absence of T cells did not positively affect DC survival (greater than 80% DC loss until d16 of culture under both conditions; Figure 1F; data not shown). Thus, MHC-dependent T-cell receptor (TCR) triggering is required and sufficient to condition T cells to elaborate DC survival-promoting signals.
The ligation of CD40 by CD40L may be a critical event during cognate DC–T-cell interaction.22 Strikingly, the specific blockade of CD40L-CD40 interaction by anti-CD40L mAbs abolished T cell–mediated DC survival (Figure 1F). As a control, recombinant CD40L added to DC cultures strongly promoted DC survival (Figure 1G). Thus, cognate T-cell encounter extends the DC lifespan in a largely CD40L-dependent fashion.
T-cell–mediated CD40 triggering selectively augments transcriptional activity of NF-κB and AP-1 in DCs
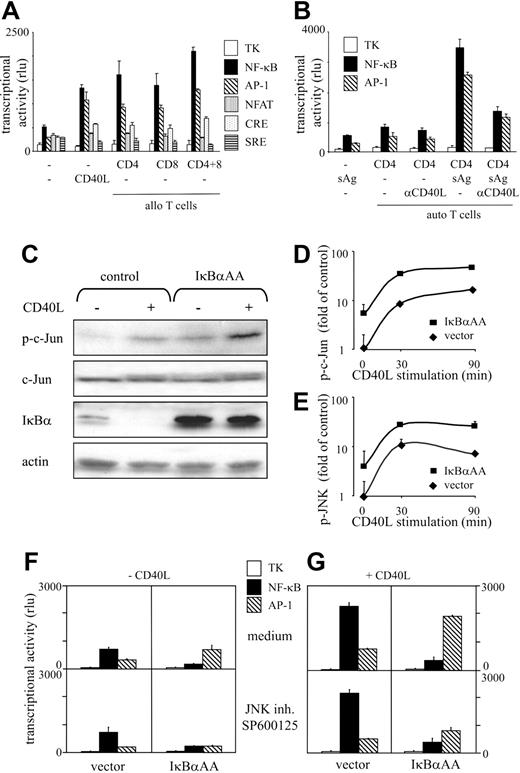
To disclose transcriptional pathways involved in the regulation of DC survival, we transfected DCs with reporter constructs monitoring NF-κB–, AP-1–, NFAT-, CRE-, or SRE–dependent gene transcription. Low constitutive transcriptional activity was detected in DCs with all constructs (Figure 2A). We observed 3-fold increased AP-1 and 3-fold increased NF-κB promoter activity when DCs were exposed to CD40L or were cocultured with allogeneic T cells (Figure 2A). A modest up-regulation of CRE activity was observed in response to CD40L and T-cell contact, whereas the transcriptional activities from SRE and NFAT elements remained unchanged (Figure 2A). Fivefold increased AP-1 and 5-fold increased NF-κB activities were observed after sAg-dependent DC–T-cell interaction (Figure 2B). The simultaneous and quantitatively balanced induction of the 2 transcriptional activities by T cells largely depended on CD40 triggering, as revealed by mAb-blocking studies (Figure 2B). To visualize the activation of candidate upstream signaling elements of the 2 pathways, we used Western blot analysis to examine the phosphorylation status of c-Jun and the degradation of IκBα in response to CD40L. As shown in Figure 2C, c-Jun is phosphorylated and IκBα is completely degraded on CD40 triggering. FACS analyses of the phosphorylation status of c-Jun (Figure 2D) and JNK (Figure 2E) revealed that both elements were activated by CD40L with similar kinetics and magnitude. Thus, JNK-dependent AP-1 and IκBα-regulated NF-κB activities are induced to an equal extent by signals that positively regulate DC survival. Hence, enhanced DC longevity correlates with a balanced induction of these 2 transcriptional pathways.
IκBαAA transfection blocks NF-κB function and augments JNK/AP-1 activity
To address the relative impact of NF-κB and AP-1 for DC survival, we next down-regulated their activities in a selective fashion. To inhibit NF-κB activity, the phosphorylation-deficient mutant IκBαAA was transfected into DCs. As shown in Figure 2C, IκBαAA transfection resulted in significant protein levels of this NF-κB super repressor. As expected, IκBαAA was not degraded after CD40 ligation. Importantly, IκBαAA transfection led to an approximately 5-fold induction of c-Jun phosphorylation (Figure 2C-D) without altering total c-Jun levels (Figure 2C). To address the contribution of JNK signaling to c-Jun phosphorylation, we used the adenosine triphosphate (ATP)–competitive inhibitor SP600125, which selectively interferes with JNK activity but not with the activity of a wide variety of other kinases.23 JNK was identified as a critical NF-κB–regulated kinase because IκBαAA transfection resulted in JNK hyperphosphorylation (Figure 2E) and SP600125 abolished c-Jun phosphorylation (data not shown). CD40 ligation further boosted JNK and c-Jun phosphorylation after IκBαAA transfection (Figure 2C-E). Transcriptional competence of IκBαAA-induced JNK/c-Jun activation was shown in reporter assays. AP-1 transcriptional activity was increased by IκBαAA expression under constitutive (Figure 2F) and CD40L-induced conditions (Figure 2G). AP-1 transcriptional activity was reduced by the JNK-specific inhibitor SP600125 at 1 μM to approximately 30% of control values in resting and in CD40-stimulated IκBαAA-transfected DCs (Figure 2F-G). In contrast, SP600125 did not alter NF-κB transcriptional activity (Figure 2F-G). Thus, IκBαAA transfection and exposure to SP600125 are effective measures to selectively inhibit NF-κB and AP-1 transcriptional activity in DCs. The down-regulation of NF-κB activity in DCs leads to the hyperinduction of JNK-dependent AP-1 activity.
Feedback-regulated NF-κB and AP-1 transcriptional activities are selectively induced in DCs on cognate T-cell contact and CD40 triggering. (A-B) Profiling of transcriptional activity. DCs were cotransfected on d11.5 with reporter constructs for the indicated transcription factors or plasmids containing the TK promoter together with pIRES2-EGFP. EGFP+/CD1a+ DCs were FACS-sorted and cultured alone and in the presence of allogeneic (A) or autologous (B) T cells. Allogeneic CD8+ and CD4+ T cells used in panel A were isolated from MNCs of the same donor. The DC/T-cell ratio was 1:100. DCs were cultured in the presence of CD40L(A) or were pulsed with TSST-1 or cultured in the presence of T cells or blocking anti-CD40L mAbs (B). Cell-free supernatants were harvested after 36 hours, and hPAP-dependent luminescence was measured. Results are expressed as mean relative light unit (rlu) (y-axis), as obtained in quadruplicate cultures. rlu values produced by mock-transfected DCs and by DCs transfected with promoterless plasmids were negligible. Reporter activity of the TK control plasmid was similar under all conditions tested. Error bars indicate SD. Results are representative of at least 3 independent determinations. (C) IκBαAA augments basal and CD40L-induced c-Jun phosphorylation but not total c-Jun levels. FACS-purified IκBαAA or control-transfected DCs were stimulated with CD40L for 30 minutes or left untreated. Cells were lysed, and extracts prepared from equal cell numbers were separated by SDS-PAGE. After protein transfer, nitrocellulose membranes were probed with the indicated antibodies. Finally, membranes were stripped and reprobed with anti–actin antibodies to ensure equal loading and transfer of cellular proteins. (D-E) IκBαAA augments basal and CD40L-induced JNK and c-Jun phosphorylation. DCs were transfected with IκBαAA or control vector and were stimulated for the indicated time periods with CD40L. Cells were washed, fixed, and permeabilized for intracellular staining with mAbs specific for p-c-Jun (D) or p-JNK (E). Data are presented as x-fold induction of phosphorylation of indicated proteins by CD40 triggering over nonstimulated control-transfected cells. Mean ± SD of 3 independent experiments are shown. (F-G) IκBαAA impairs NF-κB function and up-regulates AP-1–mediated transcriptional activity. DCs were cotransfected with reporter constructs for AP-1 or NF-κB or TK promoter–containing plasmids together with pIRES2-EGFP (vector; left column in F-G) or IκBαAA-pIRES2-EGFP (IκBαAA; right column in F-G). EGFP+/CD1a+ DCs were FACS-sorted and cultured in the absence (F) or presence (G) of CD40L. The JNK inhibitor SP600125 (1 μM) was added (bottom row in F-G). rlu (mean ± SD, y-axis) was obtained in quadruplicate cultures. Results are representative of at least 3 independent determinations.
Feedback-regulated NF-κB and AP-1 transcriptional activities are selectively induced in DCs on cognate T-cell contact and CD40 triggering. (A-B) Profiling of transcriptional activity. DCs were cotransfected on d11.5 with reporter constructs for the indicated transcription factors or plasmids containing the TK promoter together with pIRES2-EGFP. EGFP+/CD1a+ DCs were FACS-sorted and cultured alone and in the presence of allogeneic (A) or autologous (B) T cells. Allogeneic CD8+ and CD4+ T cells used in panel A were isolated from MNCs of the same donor. The DC/T-cell ratio was 1:100. DCs were cultured in the presence of CD40L(A) or were pulsed with TSST-1 or cultured in the presence of T cells or blocking anti-CD40L mAbs (B). Cell-free supernatants were harvested after 36 hours, and hPAP-dependent luminescence was measured. Results are expressed as mean relative light unit (rlu) (y-axis), as obtained in quadruplicate cultures. rlu values produced by mock-transfected DCs and by DCs transfected with promoterless plasmids were negligible. Reporter activity of the TK control plasmid was similar under all conditions tested. Error bars indicate SD. Results are representative of at least 3 independent determinations. (C) IκBαAA augments basal and CD40L-induced c-Jun phosphorylation but not total c-Jun levels. FACS-purified IκBαAA or control-transfected DCs were stimulated with CD40L for 30 minutes or left untreated. Cells were lysed, and extracts prepared from equal cell numbers were separated by SDS-PAGE. After protein transfer, nitrocellulose membranes were probed with the indicated antibodies. Finally, membranes were stripped and reprobed with anti–actin antibodies to ensure equal loading and transfer of cellular proteins. (D-E) IκBαAA augments basal and CD40L-induced JNK and c-Jun phosphorylation. DCs were transfected with IκBαAA or control vector and were stimulated for the indicated time periods with CD40L. Cells were washed, fixed, and permeabilized for intracellular staining with mAbs specific for p-c-Jun (D) or p-JNK (E). Data are presented as x-fold induction of phosphorylation of indicated proteins by CD40 triggering over nonstimulated control-transfected cells. Mean ± SD of 3 independent experiments are shown. (F-G) IκBαAA impairs NF-κB function and up-regulates AP-1–mediated transcriptional activity. DCs were cotransfected with reporter constructs for AP-1 or NF-κB or TK promoter–containing plasmids together with pIRES2-EGFP (vector; left column in F-G) or IκBαAA-pIRES2-EGFP (IκBαAA; right column in F-G). EGFP+/CD1a+ DCs were FACS-sorted and cultured in the absence (F) or presence (G) of CD40L. The JNK inhibitor SP600125 (1 μM) was added (bottom row in F-G). rlu (mean ± SD, y-axis) was obtained in quadruplicate cultures. Results are representative of at least 3 independent determinations.
Increased oxidative stress is responsible for augmented JNK/AP-1 activity in NF-κB–deficient DCs
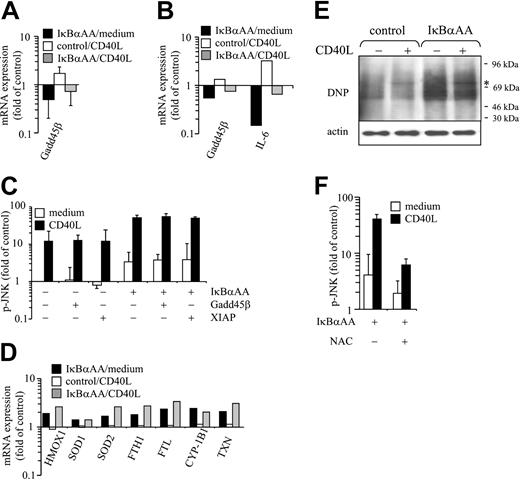
Next we investigated the mechanism by which NF-κB controls JNK activity in DCs. IκBαAA- and control-transfected DCs were stimulated or were not stimulated with CD40L and were subjected to qPCR and genome-wide RNA expression profiling. Gadd45β and API3/XIAP, the 2 NF-κB–regulated moieties previously shown to down-regulate JNK activity in murine fibroblasts,24,25 were analyzed first. Gadd45β was expressed in DCs, but its expression level was only slightly attenuated in NF-κB–deficient DCs, as revealed in parallel by qPCR (Figure 3A) and microarray analyses (Figure 3B). In contrast, interleukin-6 (IL-6) transcripts were decreased drastically by IκBαAA transfection (Figure 3B). XIAP was not expressed at significant levels in IκBαAA- or control-transfected DCs (data not shown). To determine whether the moderate reduction of Gadd45β expression in IκBαAA transfectants could still account for up-regulated JNK activity, we overexpressed Gadd45β in IκBαAA- and control-transfected DCs and investigated JNK phosphorylation with and without CD40L stimulation. As shown in Figure 3C, pJNK levels were not affected by Gadd45β (or by XIAP) overexpression. Thus, it appears that Gadd45β and XIAP are not the relevant repressors of JNK activity in human DCs.
In searching for other candidate mechanisms, we screened our microarray data for NF-κB–regulated genes that could be assigned to functional clusters. Of note, a multitude of reactive oxygen species (ROS)–regulated and –regulatory transcripts were identified within a cluster that contained genes expressed to an enhanced degree after IκBαAA transfection but not regulated, or only minimally regulated, by CD40L stimulation (Figure 3D). These include indicators of oxidative stress, such as hemoxygenase 1 (HMOX 1), antioxidant proteins such as superoxide dismutases (SODs), thioredoxin (TXN), and ferritin heavy chains (FTH1) and ferritin light chains (FTL), and radical donors such as cytochrome P450 (CYP) 1B1. We next determined the content of cellular proteins oxidized because of ROS exposure in DCs. As shown in Figure 3E, ROS-modified (carbonylated) proteins are far more abundant in NF-κB–deficient DCs than in control-transfected DCs. This strongly indicates that NF-κB is required to limit ROS loads in DCs. To investigate a possible direct role of ROS for JNK hyperactivation in NF-κB–deficient DCs, we experimentally attenuated ROS loads in DCs by N-acetyl-L-cysteine treatment. As shown in Figure 3F, the quenching of ROS in NF-κB–deficient DCs reduced constitutive and CD40L-induced JNK hyperactivation by more than 50%. Thus, NF-κB deficiency results in an increased generation of ROS, which, in turn, up-regulates JNK/AP-1 activity in DCs.
Oxidative stress accounts for JNK up-regulation in NF-κB–deficient DCs. (A-B,D) DCs were transfected with pIRES2-EGFP or IκBαAA-pIRES2-EGFP, FACS-sorted, and cultured in the absence or presence of CD40L for 6 hours. RNA was extracted and either (A) subjected to qPCR analysis or (B,D) processed for microarray analysis. (D) Transcripts investigated included those for HMOX1, SOD1 and SOD2, FTH1, FTL, CYP-1B1, and TXN. Data are presented as x-fold induction of respective transcripts over values obtained from control-transfected, nonstimulated DCs. (A) Mean ± SD of 4 independent experiments. (C) Overexpression of XIAP or Gadd45β does not impair up-regulation of p-JNK in IκBαAA-transfected DCs. DCs were transfected with indicated vectors and stimulated for 30 minutes with CD40L. Cells were washed, fixed, and permeabilized for intracellular staining with mAbs specific for p-JNK. Data are presented as x-fold induction of JNK phosphorylation over JNK phosphorylation of nonstimulated, control-transfected cells. Mean ± SD of 3 independent experiments. (E-F) Augmented oxidative stress accounts for JNK induction in NF-κB–deficient DCs. Transfected DCs were sorted and stimulated for 30 minutes with CD40L. Cells were lysed, and proteins were derivatized for the detection of carbonylation, as described in “Materials and methods.” Proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes. (E) Blots were probed with DNP-specific antibodies. Strikingly augmented levels of carbonylated proteins were detected in IκBαAA-transfected DCs. Minor qualitative differences in the pattern of oxidized proteins were observed between CD40L-stimulated and nonstimulated cells. *Band of approximately 75 kDa that selectively appeared on CD40 ligation. (F) Cells preincubated for 30 minutes with N-acetyl-L-cysteine (NAC; final concentration, 60 mM) or medium only were stimulated or were not stimulated with CD40L for 30 minutes and were subjected to p-JNK immunostaining and FACS analysis. Mean ± SD of 3 independent experiments are shown.
Oxidative stress accounts for JNK up-regulation in NF-κB–deficient DCs. (A-B,D) DCs were transfected with pIRES2-EGFP or IκBαAA-pIRES2-EGFP, FACS-sorted, and cultured in the absence or presence of CD40L for 6 hours. RNA was extracted and either (A) subjected to qPCR analysis or (B,D) processed for microarray analysis. (D) Transcripts investigated included those for HMOX1, SOD1 and SOD2, FTH1, FTL, CYP-1B1, and TXN. Data are presented as x-fold induction of respective transcripts over values obtained from control-transfected, nonstimulated DCs. (A) Mean ± SD of 4 independent experiments. (C) Overexpression of XIAP or Gadd45β does not impair up-regulation of p-JNK in IκBαAA-transfected DCs. DCs were transfected with indicated vectors and stimulated for 30 minutes with CD40L. Cells were washed, fixed, and permeabilized for intracellular staining with mAbs specific for p-JNK. Data are presented as x-fold induction of JNK phosphorylation over JNK phosphorylation of nonstimulated, control-transfected cells. Mean ± SD of 3 independent experiments. (E-F) Augmented oxidative stress accounts for JNK induction in NF-κB–deficient DCs. Transfected DCs were sorted and stimulated for 30 minutes with CD40L. Cells were lysed, and proteins were derivatized for the detection of carbonylation, as described in “Materials and methods.” Proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes. (E) Blots were probed with DNP-specific antibodies. Strikingly augmented levels of carbonylated proteins were detected in IκBαAA-transfected DCs. Minor qualitative differences in the pattern of oxidized proteins were observed between CD40L-stimulated and nonstimulated cells. *Band of approximately 75 kDa that selectively appeared on CD40 ligation. (F) Cells preincubated for 30 minutes with N-acetyl-L-cysteine (NAC; final concentration, 60 mM) or medium only were stimulated or were not stimulated with CD40L for 30 minutes and were subjected to p-JNK immunostaining and FACS analysis. Mean ± SD of 3 independent experiments are shown.
IκBαAA transfection converts T-cell– and CD40L-mediated DC survival signals to death signals
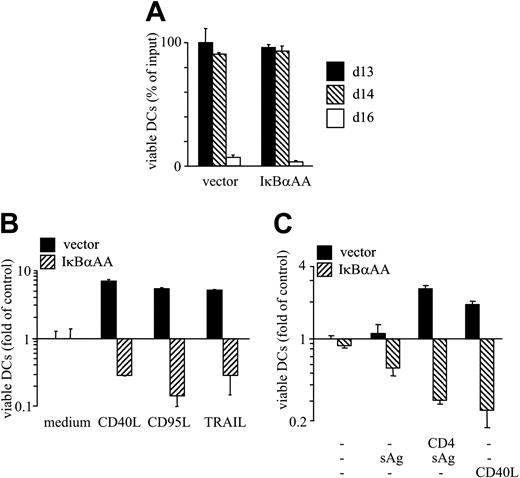
Next we determined whether experimental impairment of AP-1 or NF-κB transcriptional activity affects DC viability. IκBαAA- and control-transfected DCs underwent cell death with similar kinetics and similar magnitude (Figure 4A). Biologic effects of NF-κB deficiency became evident when DCs were exposed to CD40L (Figure 4B-C), CD95L, TRAIL (Figure 4B), or autologous T cells plus sAg (Figure 4C). In all these situations, massive DC death was observed. Conversely, NF-κB–activating TNF superfamily (TNF-SF) members CD40L, CD95L, TRAIL,6,26-28 and cognate T-cell interaction promoted the survival of control-transfected DCs in the same experiment (Figure 4B-C). Thus, NF-κB activity in DCs controls the decision whether signals transduced by the TNF receptor superfamily (TNFR-SF) members are perceived as survival or as death signals.
Loss of NF-κB activity in DCs converts antiapoptotic signals to proapoptotic signals. (A-C) DCs were transfected with pIRES2-EGFP or IκBαAA-pIRES2-EGFP, and CD1a+/EGFP+ DCs were FACS-sorted. (A) DCs were cultured without stimulants, and viable cells were enumerated at the indicated time points of culture. (B) DCs were cultured in the presence or absence of CD40L, CD95L, or TRAIL. (C) Native or sAg-pulsed DCs were cultured in the presence of autologous T cells or CD40L. (B-C) Viable DCs were enumerated on d15 of culture. Results are presented as percentage of viable DCs after culture relative to the number of input DCs (mean ± SD of triplicate determinations) (A) or as x-fold alteration of survival (mean ± SD of triplicate experiments) relative to the survival of vector-transfected DCs cultured in medium only (B-C). Results are representative of at least 3 independent experiments.
Loss of NF-κB activity in DCs converts antiapoptotic signals to proapoptotic signals. (A-C) DCs were transfected with pIRES2-EGFP or IκBαAA-pIRES2-EGFP, and CD1a+/EGFP+ DCs were FACS-sorted. (A) DCs were cultured without stimulants, and viable cells were enumerated at the indicated time points of culture. (B) DCs were cultured in the presence or absence of CD40L, CD95L, or TRAIL. (C) Native or sAg-pulsed DCs were cultured in the presence of autologous T cells or CD40L. (B-C) Viable DCs were enumerated on d15 of culture. Results are presented as percentage of viable DCs after culture relative to the number of input DCs (mean ± SD of triplicate determinations) (A) or as x-fold alteration of survival (mean ± SD of triplicate experiments) relative to the survival of vector-transfected DCs cultured in medium only (B-C). Results are representative of at least 3 independent experiments.
JNK/AP-1 signaling executes death of IκBαAA-transfected DCs
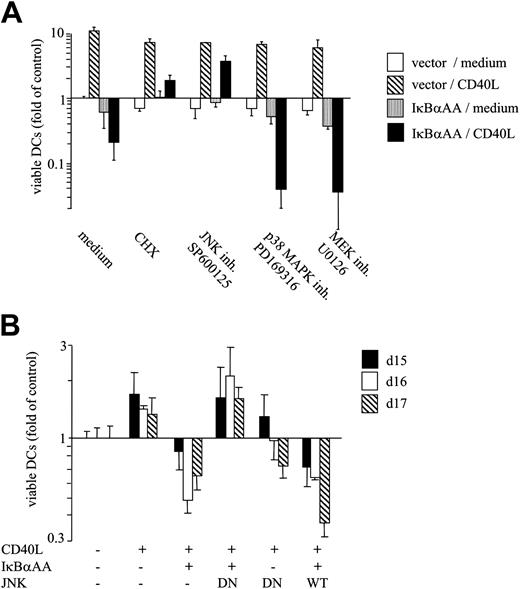
We next addressed whether JNK/AP-1 activity superinduced by the lack of NF-κB control is functionally relevant for DC apoptosis. In the first series of experiments, pharmacologic inhibition of JNK activity was found to abolish CD40-mediated apoptosis and to restore CD40-dependent survival in NF-κB–deficient DCs (Figure 5A). Importantly, the survival-promoting effect of JNK inhibition occurred selectively in IκBαAA-transfected DCs but not in vector-transfected DCs (Figure 5A). Inhibition of protein synthesis similarly resulted in the rescue of IκBαAA-transfected DCs, indicating a relevant contribution of JNK/AP-1 transcriptional activity for active DC death. Inhibiting critical checkpoints of other stress kinase pathways (p38 MAPK and ERK/MEK) did not rescue DC survival but augmented DC death (Figure 5A).
In a second series of experiments, inhibition of JNK activity was achieved by transfecting a DN N-terminal JNK deletion mutant lacking the ATP-binding site.19 As expected, CD40 ligation enhanced DC survival in control-transfected DCs but induced DC death in IκBαAA-transfected DCs (Figure 5B). Cotransfection of DN-JNK completely rescued DCs from CD40L-induced apoptosis (Figure 5B). As seen in pharmacologic JNK inhibition experiments, DN-JNK also restored CD40L-mediated survival (Figure 5B). As a control, the transfection of WT-JNK did not prevent apoptosis of NF-κB–deficient DCs. Thus, our data show that JNK can be a critical mediator of DC death. It is important to note that, in the presence of functional NF-κB, DN-JNK and SP600125 did not promote DC survival but remained neutral or even slightly increased DC death (Figure 5A-B). Thus, we conclude that only a balanced activation of the JNK/AP-1 and the NF-κB signaling pathways results in enhanced DC longevity. Imbalanced JNK/AP-1 activation resulting from NF-κB deficiency promotes DC apoptosis.
Freshly isolated human epidermal Langerhans cells require balanced activation of JNK and NF-κB to survive
Thus far, results were obtained with in vitro–generated human HPC-derived DCs that closely resemble epidermal LCs in vivo. To unequivocally define whether the postulated mechanism is of relevance in naturally occurring DCs, we isolated human epidermal LCs and transfected them with IκBαAA or control vector. LCs were cultured in the absence or presence of the pharmacologic JNK inhibitor and were activated or were not activated by CD40L (Figure 6A). In striking similarity to HPC-derived DCs, NF-κB–deficient LCs were efficiently killed by CD40L stimulation but survived in the absence of CD40L (Figure 6A). The only moderate survival-promoting effect of CD40L in control transfectants can be attributed to efficient spontaneous survival of the purified LCs (more than 70% of input LCs were still alive after 24 hours in the absence of CD40L). CD40L-induced LC death was abolished, and CD40L-mediated survival was reintroduced by JNK inhibition, as observed in HPC-derived DCs (Figure 6A). Thus, JNK and NF-κB can reciprocally regulate the life and death of a physiologically important DC subset isolated ex vivo, strongly supporting the biologic relevance of feedback-balanced transcription factor activation for DC survival in vivo.
Overexpression of mitochondria-related antiapoptotic proteins can rescue DCs from JNK-mediated death
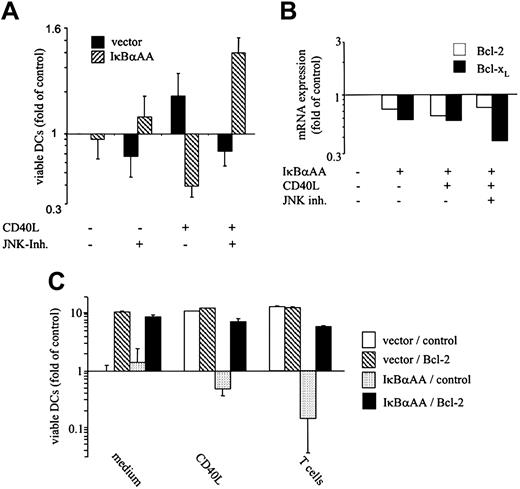
To test whether death execution by NF-κB deficiency–associated, ROS-mediated JNK activation may involve the down-regulation of prosurvival factors, we measured the effect of IκBαAA expression in DCs on the regulation of antiapoptotic proteins of the Bcl family that maintain mitochondrial integrity (ie, Bcl-2, Bcl-xL).29,30 IκBαAA transfection resulted in moderate (approximately 30%-40%) reduction of Bcl-2 and Bcl-xL at the mRNA (Figure 6B) and the protein levels (data not shown). CD40 stimulation and inhibition of JNK activity did not reverse this expression deficit (Figure 6B). Thus, Bcl down-regulation is a direct consequence NF-κB deficiency but is not caused by ROS-mediated JNK hyperactivation. More important, Bcl family member expression was down-regulated to the same extent under conditions that sufficed for DC survival (IκBαAA transfection) and that resulted in JNK-mediated DC death (IκBαAA transfection plus CD40 stimulation; Figure 6B). Thus, the down-regulation of Bcl expression does not directly contribute to DC death, yet it may be a cofactor that sensitizes DCs for JNK-mediated apoptosis. To investigate this issue further, we overexpressed Bcl proteins or proteins that modulated the activation of caspases and assessed their capacity to rescue DCs from JNK-mediated cell death. Constructs summarized in Table 1 were cotransfected into DCs, together with control vectors or vectors coding for IκBαAA, and constitutive, CD40L-, and T-cell–mediated DC survival was measured. All 3 Bcl family members tested (Bcl-2, Bcl-xL, A131 ) effectively rescued IκBαAA-transfected DCs from JNK/AP-1–dependent apoptosis (Figure 6C; Table 1). Unlike JNK inhibition (Figure 5), the overexpression of Bcl members also strongly increased the constitutive survival of control vector-transfected DCs (Figure 6C). In aggregate, Bcl family members can effectively counteract spontaneous and forced DC death, but their down-regulation caused by NF-κB deficiency does not directly contribute to the execution of DC death by ROS-induced JNK, an argument further supported by the almost complete restoration of DC survival by JNK inhibition (Figure 5). Unlike Bcl proteins, immediate early gene-1 (IEX-1) and cellular FLICE inhibitory protein (cFLIP) were only moderately effective in counteracting JNK-dependent apoptosis. A20, Gadd45β, and proteins belonging to the apoptosis inhibitor/human inhibitor of apoptosis (API/HIAP) family—that is, API1/HIAP2, API2/HIAP1, API3/X-linked IAP-like protein (XIAP), and API4/survivin—were entirely ineffective in this regard (Table 1). Pharmacologic inhibition of caspase-3 but, less so, of caspase-8 also resulted in effective rescue of DCs from apoptosis (Table 1). Thus, we conclude that the main death mechanism initiated by JNK/AP-1 likely entails a mitochondrial pathway of apoptosis that culminates in caspase-3 activation.32
Blockade of JNK/AP-1 activation and protein neosynthesis restores CD40-mediated survival of NF-κB–deficient DCs. (A) Pharmacologic inhibition of JNK/AP-1 activation, but not of p38 MAPK or MEK/ERK function, restores CD40L-dependent survival in NF-κB–deficient DCs. DCs were transfected with pIRES2-EGFP or IκBαAA-pIRES2-EGFP. FACS-sorted transfectants were cultured for 3 days in the absence or presence of CD40L. Inhibitors were used at 10 μg/mL (cycloheximide, CHX) or 1 μM (SP600125, PD169316, U0126). (B) Transfection of DN-JNK restores CD40L-dependent survival in NF-κB–deficient DCs. DCs were transfected with IκBαAA or control vector together with plasmids encoding DN-JNK, WT-JNK, or empty control vector on day 11.5. DCs were sorted and reseeded on day 12, exposed to CD40L or medium only, and cultured for the indicated time periods. (A-B) Results are presented as mean x-fold alteration of survival (mean ± SD of triplicate experiments) relative to the survival of vector-transfected DCs cultured in medium only. (B) Data were normalized to control cells for each individual time point presented. Results are representative of at least 3 independent experiments.
Blockade of JNK/AP-1 activation and protein neosynthesis restores CD40-mediated survival of NF-κB–deficient DCs. (A) Pharmacologic inhibition of JNK/AP-1 activation, but not of p38 MAPK or MEK/ERK function, restores CD40L-dependent survival in NF-κB–deficient DCs. DCs were transfected with pIRES2-EGFP or IκBαAA-pIRES2-EGFP. FACS-sorted transfectants were cultured for 3 days in the absence or presence of CD40L. Inhibitors were used at 10 μg/mL (cycloheximide, CHX) or 1 μM (SP600125, PD169316, U0126). (B) Transfection of DN-JNK restores CD40L-dependent survival in NF-κB–deficient DCs. DCs were transfected with IκBαAA or control vector together with plasmids encoding DN-JNK, WT-JNK, or empty control vector on day 11.5. DCs were sorted and reseeded on day 12, exposed to CD40L or medium only, and cultured for the indicated time periods. (A-B) Results are presented as mean x-fold alteration of survival (mean ± SD of triplicate experiments) relative to the survival of vector-transfected DCs cultured in medium only. (B) Data were normalized to control cells for each individual time point presented. Results are representative of at least 3 independent experiments.
Effects of antiapoptotic constructs and pharmacologic inhibitors on CD40L- and T-cell—mediated death of IκBαAA-transfected DCs
. | Prevention of death mediated by . | . | |
|---|---|---|---|
. | CD40L . | T cells plus sAg . | |
| Constructs* | |||
| A1 | ++ | ++ | |
| Bcl-2 | ++ | ++ | |
| Bcl-xL | ++ | ++ | |
| cFlip | + | + | |
| IEX-1 | + | + | |
| API1/HIAP2 | - | - | |
| API2/HIAP1 | - | - | |
| API3/XIAP | - | - | |
| AP14/survivin | - | - | |
| A20 | - | - | |
| Gadd45β | - | ND | |
| Pharmacologic inhibitors† | |||
| Caspase-3 inh. | ++ | ND | |
| Caspase-8 inh. | - | ND | |
. | Prevention of death mediated by . | . | |
|---|---|---|---|
. | CD40L . | T cells plus sAg . | |
| Constructs* | |||
| A1 | ++ | ++ | |
| Bcl-2 | ++ | ++ | |
| Bcl-xL | ++ | ++ | |
| cFlip | + | + | |
| IEX-1 | + | + | |
| API1/HIAP2 | - | - | |
| API2/HIAP1 | - | - | |
| API3/XIAP | - | - | |
| AP14/survivin | - | - | |
| A20 | - | - | |
| Gadd45β | - | ND | |
| Pharmacologic inhibitors† | |||
| Caspase-3 inh. | ++ | ND | |
| Caspase-8 inh. | - | ND | |
Modified DCs were cultured in the absence or in the presence of CD40L or autologous CD4+ T cells plus sAg, and viable DCs were counted 3 days later using FACS. Survival of control-transfected DCs and of IκBαAA-transfected DCs in the presence of CD40L or T cells was set to 100% and 0%, respectively. (++) Rescue of survival of 50% or more to 100%, (+) of 10% or more to 50%, and (-) of less than 10% in CD40L-exposed (left column) and T cell/sAg-exposed (right column) DCs. Results are representative of 2 to 4 independent experiments.
ND indicates not determined.
Indicated transgenes or empty vector only were cotransfected with IκBαAA or control vector.
Alternatively, IκBαAA- or control vector—transfected DCs were incubated with the indicated caspase inhibitors (20 μM each).
JNK/AP-1–mediated death in freshly isolated epidermal Langerhans cells, regulation of Bcl transcripts, and reversal of JNK/AP-1–induced DC death by Bcl family members. (A) Inhibition of JNK/AP-1 activation restores CD40-mediated survival in IκBαAA-transfected primary skin LCs. Epidermal cells containing 10% to 20% CD1a+ LCs were transfected with pIRES2-EGFP or IκBαAA-pIRES2-EGFP. Twelve hours after transfection, JNK inhibitor SP600125 (1 μM) was added where indicated; 30 minutes later, cells were exposed to CD40L or were left unstimulated. Cells were cultured for 24 hours and then were labeled with CD1a-PE and propidium iodide (PI). Viable transfected EGFP+/CD1a+/FSChigh DCs that excluded PI were enumerated by FACS. Data are shown as x-fold alteration of survival (mean ± SD of 4 independent experiments) relative to the survival of vector-transfected LCs cultured in medium only. (B) DCs were transfected with pIRES2-EGFP or IκBαAA-pIRES2-EGFP, FACS-sorted, and cultured in the absence or presence of CD40L or JNK inhibitor SP600125 for 6 hours. RNA was extracted and processed for microarray analysis. Relative expression of Bcl-2 and Bcl-xL transcripts is shown. (C) Induced Bcl-2 expression restores CD40-mediated survival in NF-κB–deficient, HPC-derived DCs. DCs were cotransfected with Bcl-2 or empty vector (control) together with pIRES2-EGFP (vector) or IκBαAA-pIRES2-EGFP (IκBαAA). CD1a+/EGFP+ DCs were FACS-sorted and exposed to the indicated stimuli for 3 days. (A,C) Data are shown as x-fold alteration of survival (mean ± SD of 3 independent experiments) relative to the survival of vector-transfected DCs cultured in medium only.
JNK/AP-1–mediated death in freshly isolated epidermal Langerhans cells, regulation of Bcl transcripts, and reversal of JNK/AP-1–induced DC death by Bcl family members. (A) Inhibition of JNK/AP-1 activation restores CD40-mediated survival in IκBαAA-transfected primary skin LCs. Epidermal cells containing 10% to 20% CD1a+ LCs were transfected with pIRES2-EGFP or IκBαAA-pIRES2-EGFP. Twelve hours after transfection, JNK inhibitor SP600125 (1 μM) was added where indicated; 30 minutes later, cells were exposed to CD40L or were left unstimulated. Cells were cultured for 24 hours and then were labeled with CD1a-PE and propidium iodide (PI). Viable transfected EGFP+/CD1a+/FSChigh DCs that excluded PI were enumerated by FACS. Data are shown as x-fold alteration of survival (mean ± SD of 4 independent experiments) relative to the survival of vector-transfected LCs cultured in medium only. (B) DCs were transfected with pIRES2-EGFP or IκBαAA-pIRES2-EGFP, FACS-sorted, and cultured in the absence or presence of CD40L or JNK inhibitor SP600125 for 6 hours. RNA was extracted and processed for microarray analysis. Relative expression of Bcl-2 and Bcl-xL transcripts is shown. (C) Induced Bcl-2 expression restores CD40-mediated survival in NF-κB–deficient, HPC-derived DCs. DCs were cotransfected with Bcl-2 or empty vector (control) together with pIRES2-EGFP (vector) or IκBαAA-pIRES2-EGFP (IκBαAA). CD1a+/EGFP+ DCs were FACS-sorted and exposed to the indicated stimuli for 3 days. (A,C) Data are shown as x-fold alteration of survival (mean ± SD of 3 independent experiments) relative to the survival of vector-transfected DCs cultured in medium only.
Discussion
Enhancement of DC longevity can profoundly augment the magnitude of cellular immune responses.6,13,33 Conversely, premature induction of DC death likely prevents the occurrence of T-cell activation and expansion.1 Although pathways positively influencing the DC lifespan are thus partly understood, the signals that actively mediate DC death remain elusive. A simple surface receptor–based model of survival regulation does not apply to DCs because prototype death receptors can activate DCs rather than kill these cells.9 Abundant expression in DCs of cFLIP that prevents caspase activation through death domain–containing surface receptors was evidenced to contribute to this phenomenon.34,35 In this study, we asked whether a consensus concept of the regulation of DC longevity and death might unfold at the level of signal transduction. We could functionally link specific patterns of transcription factor activation to whether a DC survives or dies. We further suggest that the balance of transcription factors activated by DC surface receptors, but not necessarily the type of the triggered receptor, decides the fate of a DC.
To systematically screen for the induction of biologically important transcriptional pathways, DCs were transfected with a panel of appropriate reporter constructs and analyzed during surrogate antigen-dependent T-cell conjugation and after triggering of CD40 by CD40L. NF-κB and JNK/AP-1 were the transcriptional activators most pronouncedly up-regulated in the various experimental settings. In contrast, NFAT-, CRE-, or SRE-dependent transcription remained largely unaffected. Thus, a distinctive transcription factor profile is induced during TNFR-SF member and T cell–mediated DC activation.
All TNFR-SF members investigated in this study (CD40, CD95, TRAIL-R) were able to signal survival in DCs. In this respect, our data confirm and extend previous observations.5,13,33 As a further extension, we demonstrate that CD40 is the major receptor that mediates DC survival during cognate T-cell contact and that this effect is mediated mainly by NF-κB activation. Experimentally induced NF-κB deficiency did not, however, simply result in an abolishment of TNFR-SF–mediated DC survival. In turn, cognate T-cell contact and receptor-initiated signaling actively induced the death of NF-κB-deficient DCs. This DC elimination process required simultaneous receptor triggering and protein neosynthesis and was executed by caspases. Thus, receptor-mediated DC death can be regarded as activation-induced cell death (AICD), an established pathway of lymphocyte elimination.36
The JNK/AP-1 signaling pathway acts as a multifunctional regulator of embryonic development and of cellular proliferation and differentiation in many tissues. The role of JNK/AP-1 extends to the regulation of cell survival as it functions as an antiapoptotic promoter of tumorigenesis and stress-related inducer of programmed cell death.37 The occurrence of these extremes likely depends on the cell type, the magnitude and duration of the JNK/AP-1 signal, the individual JNK/AP-1 components engaged, and the modulating effects from other concomitantly active signaling pathways.38 Here, we add another immunologically relevant function to the activities of JNK/AP-1. We show that JNK/AP-1 signaling is a major pathway of AICD of DCs. Blockade of JNK/AP-1 signaling abolished AICD and restored activation-induced survival of NF-κB–deficient HPC-derived DCs. The further observation of JNK-mediated AICD in ex vivo isolated human LCs (Figure 6A) and plasmacytoid DCs (unpublished observations, May 2004) strongly argues that the identified DC death program can operate in functionally distinctive DC subsets in vivo. In contrast, inhibition of MAPK or MEK/ERK, essential components of 2 other stress-activated signaling pathways, did not rescue DCs but promoted DC death. Thus, we conclude that the JNK/AP-1 signaling pathway can actively and selectively promote AICD of DCs in the absence of functional NF-κB.
In our study, only balanced NF-κB and JNK/AP-1 activity is associated with prolonged DC survival. Low NF-κB and high JNK/AP-1 activity signals cell death to DCs (Figure 7). Upper limits of JNK/AP-1 signaling strength in DCs are likely set by a NF-κB–dependent negative feedback loop. NF-κB–mediated negative feedback regulation of JNK on TNF-R triggering was previously identified in murine fibroblasts.24,25 XIAP and Gadd45β were candidate NF-κB targets to repress JNK activity in these cells. Transfection with either of the human orthologs failed to inhibit JNK activation and to prevent JNK/AP-1–induced cell death in human DCs in our study. Thus, these molecules either are not relevant repressors of JNK or its upstream kinases39 in DCs or are not sufficiently down-regulated in IκBαAA-transfected DCs to allow for JNK hyperactivation. In turn, our study shows that limiting ROS loads is a major NF-κB function that results in the control of JNK activity in DCs. This is directly shown by exaggerated ROS activity and ROS-mediated JNK activation in NF-κB–deficient DCs. Ferritin heavy chain, a key transcriptional NF-κB target found to repress ROS generation and JNK activation,40 was not down-regulated in NF-κB–deficient DCs (Figure 4) and, therefore, is unlikely to be involved in JNK regulation in human DCs. We thus conclude that NF-κB feedback control of JNK activity is a regulatory circuit conserved between murine stroma cells and human DCs, suggesting its wide biologic importance. However, mechanistically the molecular basis of JNK inhibition by NF-κB includes a spectrum of activities that may be used in a cell-type or a species-restricted fashion, or both.
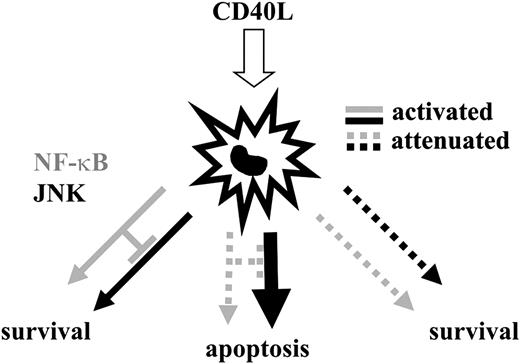
Model of the regulation of DC longevity and death by the balance of JNK/AP-1 and NF-κB activation. DCs enjoy a prolonged lifespan when JNK/AP-1 and NF-κB are activated in a balanced fashion by the ligation of TNFR-SF members. A main function of NF-κB in DCs is to restrict the amount of JNK activity. When NF-κB activation is impaired, TNFR-SF member-induced JNK activity is augmented, and DCs undergo JNK/AP-1–mediated apoptosis. If JNK/AP-1 and NF-κB activities are attenuated simultaneously, TNFR-SF member–induced survival is reestablished. Thus, a balance of JNK/AP-1 and NF-κB activity, rather than the absolute degree of activity amplification of a single pathway, mediates DC survival. Noteworthy is that this model does not rule out the possible absolute requirement of a critical NF-κB signaling threshold for DC survival.
Model of the regulation of DC longevity and death by the balance of JNK/AP-1 and NF-κB activation. DCs enjoy a prolonged lifespan when JNK/AP-1 and NF-κB are activated in a balanced fashion by the ligation of TNFR-SF members. A main function of NF-κB in DCs is to restrict the amount of JNK activity. When NF-κB activation is impaired, TNFR-SF member-induced JNK activity is augmented, and DCs undergo JNK/AP-1–mediated apoptosis. If JNK/AP-1 and NF-κB activities are attenuated simultaneously, TNFR-SF member–induced survival is reestablished. Thus, a balance of JNK/AP-1 and NF-κB activity, rather than the absolute degree of activity amplification of a single pathway, mediates DC survival. Noteworthy is that this model does not rule out the possible absolute requirement of a critical NF-κB signaling threshold for DC survival.
Identifying an inducible death pathway in DCs executed by JNK/AP-1 that is operative even in primary human cells can have substantial biologic importance. Medically relevant viral, bacterial, and protozoan pathogens that can infect DCs have evolutionarily adapted strategies to abolish NF-κB activity in host cells.41-44 For example, the HIV protein Vpu efficiently silences NF-κB by IκBα stabilization,41,45 the inhibitory strategy also followed in this study. Thus, our data show that silencing of NF-κB activity in infected host DCs can be a microbial strategy to evade immune recognition by inducing JNK/AP-1–mediated elimination of infected DCs. Conversely, selective blockade of NF-κB in DCs now appears a promising strategy to prevent sensitization to grafted organs. Effectively, NF-κB–depleted graft DCs will be killed by alloantigen-specific host T cells, and a state of immunologic ignorance or even tolerance may develop after cross-presentation of apoptotic graft DCs by host APCs.
Prepublished online as Blood First Edition Paper, March 8, 2005; DOI 10.1182/blood-2004-08-3072.
Supported by the Center of Molecular Medicine of the Austrian Academy of Sciences, the Austrian Science Foundation (FWF; SFB F2308), and the GENAU program of the Austrian Ministry of Science (D.M.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Profs Erwin F. Wagner, Syu-ichi Hirai, Jürg Tschopp, and Maria Sibilia for critical reading of the manuscript and B. Reininger for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal