Anemia occurs in more than 30% of patients with epithelial ovarian cancer before any surgery. High levels of proinflammatory cytokines and increased oxidative stress may contribute to the development of cancer-related anemia. We assessed a population of previously untreated patients with advanced epithelial ovarian cancer to evaluate whether there was a correlation between hemoglobin (Hb) and parameters of inflammation and oxidative stress, stage of disease, and performance status (PS). In 91 patients with epithelial ovarian cancer and 95 healthy women matched for age, weight, and height, levels of Hb, C-reactive protein (CRP), fibrinogen (Fbg), proinflammatory cytokines, leptin, reactive oxygen species (ROS), and antioxidant enzymes were assessed at diagnosis before treatment. The correlations between Hb, parameters of inflammation and oxidative stress, stage, and PS were evaluated. Hb levels were lower in patients with advanced epithelial ovarian cancer than in control subjects and inversely related to stage and PS. Hb negatively correlated with CRP, Fbg, interleukin 1β (IL-1β), IL-6, tumor necrosis factor α (TNFα), and ROS, and positively correlated with leptin and glutathione peroxidase (GPx). Multivariate regression analysis showed that stage and IL-6 were independent factors determining Hb values. This evidence suggests that anemia in epithelial ovarian cancer is common and its presence is related to stage of disease and markers of inflammation.
Introduction
Anemia is present in more than 30% of patients with epithelial ovarian cancer at the time of initial presentation. The severity of cancer-related anemia (CRA) has been associated with more aggressive tumors.1 Nevertheless, few studies have focused on the mechanisms of cancer-induced anemia.
CRA is typically normochromic, normocytic with a low reticulocyte count. Bone marrow iron stores are adequate or increased, but iron reutilization is impaired, as shown by normal or increased ferritin levels and low serum iron levels and iron binding capacity.2 In CRA, erythroid progenitor cells respond normally to erythropoietin (EPO), but EPO production is often not optimal for the level of anemia.3 The biologic and hematologic characteristics of CRA are similar to those observed in anemia occurring in chronic inflammatory diseases.4-7
Markers of chronic inflammation, commonly observed in patients with malignancy, are thought to relate to the stage of disease, to low performance status (PS), and to compromised nutritional status and weight loss.8-10 Inflammatory mediators, particularly cytokines (interleukin 6 [IL-6], tumor necrosis factor α [TNFα], and IL-1β) have been recognized to play a key role in inducing anorexia, nausea/vomiting, and the severe energy metabolism disorders occurring in patients with advanced cancer. The release of proinflammatory cytokines in neoplastic patients is often associated with increased production of reactive oxygen species (ROS) (H2O2 and
Several in vitro and in vivo studies demonstrated that high levels of proinflammatory cytokines and increased oxidative stress contribute both to the development of anemia and to the resistance to recombinant human EPO.14 Therefore, we asked, in the absence of blood loss, myelosuppressive therapy, and bone metastases, whether the same inflammatory substances, known for their role in the onset of cachexia syndrome, could account for the development of CRA.
Many studies have shown a correlation between inflammation, elevated circulating cytokines, and anemia in mice and in patients with chronic inflammation or end-stage renal disease,15 but direct evidence for this association in a wide population of patients with cancer is lacking. Accordingly, we assessed a population of previously untreated women with epithelial ovarian carcinoma to determine the relationship of hemoglobin (Hb) and parameters of inflammatory stress, including cytokines and ROS.
Patients and methods
The study, approved by the local Ethical Committee and by the Institutional Review Board, was carried out at the Department of Obstetrics and Gynecology, Sirai Hospital, Carbonia; and the Department of Obstetrics and Gynecology and Department of Medical Oncology of the University of Cagliari, Cagliari, Italy. The study was performed in accordance with the Declaration of Helsinki. All subjects participated in the study as volunteers, after signing an informed written consent.
The subjects included in the study were 91 women with epithelial ovarian cancer (mean age, 62.1 years; range, 45-81 years) (study group), and 95 healthy women matched for age, weight, and height (mean age, 61 years; range, 46-78 years) (control group). The clinical characteristics of the patients with epithelial ovarian cancer and the control subjects are reported in Table 1. The diagnosis of ovarian cancer was histologically confirmed in all patients. The stage of ovarian cancer was carried out in accordance to the International Federation of Gynecology and Obstetrics classification system. At the time of study, no patient had received surgery, radiotherapy, chemotherapy, or other medical interventions. No subject included in the study had evidence of infections, gastrointestinal disease, vaginal bleeding, or hemolysis.
Clinical characteristics of patients with epithelial ovarian cancer
. | Patients . | Control subjects . |
|---|---|---|
| No. | 91 | 95 |
| Mean age, y (range) | 62.1 (45-81) | 61.0 (46-78) |
| Mean weight, kg (range) | 59.0 (37-85) | 62.4 (45-76) |
| Mean height, cm (range) | 156.7 (140-165) | 157.8 (145-168) |
| Tumor sites, no. (%) | ||
| Ovary | 91 (100) | NA |
| Tumor stage, no. (%) | ||
| I-II | 25 (27.5) | NA |
| III-IV | 66 (72.5) | NA |
| ECOG PS, no. (%) | ||
| 0 | 15 (16.4) | NA |
| 1 | 27 (29.7) | NA |
| 2 | 36 (39.6) | NA |
| 3 | 13 (14.3) | NA |
. | Patients . | Control subjects . |
|---|---|---|
| No. | 91 | 95 |
| Mean age, y (range) | 62.1 (45-81) | 61.0 (46-78) |
| Mean weight, kg (range) | 59.0 (37-85) | 62.4 (45-76) |
| Mean height, cm (range) | 156.7 (140-165) | 157.8 (145-168) |
| Tumor sites, no. (%) | ||
| Ovary | 91 (100) | NA |
| Tumor stage, no. (%) | ||
| I-II | 25 (27.5) | NA |
| III-IV | 66 (72.5) | NA |
| ECOG PS, no. (%) | ||
| 0 | 15 (16.4) | NA |
| 1 | 27 (29.7) | NA |
| 2 | 36 (39.6) | NA |
| 3 | 13 (14.3) | NA |
NA indicates not applicable; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
In all subjects the body mass index (BMI) was calculated as weight (in kg)/height (in m2), whereas the PS was quantified using the World Health Organization–approved Eastern Cooperative Oncology Group (ECOG) PS scale.16 This scale evaluates 3 dimensions of health status simultaneously (activity, work, self-care), and scores range from 0 (fully active) to 5 (dead).
In all subjects a blood sample was obtained from venipuncture of an antecubital vein at 8 AM after overnight fasting. The blood samples were collected in tubes with clot-activating factor and centrifuged immediately after collection, and serum was stored at -20°C until assayed. In each serum sample the levels of Hb, C-reactive protein (CRP), fibrinogen (Fbg), proinflammatory cytokines (IL-6, IL-1, TNFα), IL-2, and leptin were analyzed. ROS and antioxidant enzymes (glutathione peroxidase [GPx], and superoxide dismutase [SOD]) were assessed on fresh heparinized blood samples.
Assessment of Hb, hematologic, and iron parameters (RBC, MCV, MCHC, absolute reticulocyte count, serum iron, and ferritin)
All control subjects and patients with ovarian cancer were subjected to the following analysis: Hb concentration, red blood cell (RBC) count, mean cell volume (MCV), mean cell hemoglobin concentration (MCHC), reticulocyte count (percentage and absolute number), concentration of serum iron, and ferritin. Red blood cell count and reticulocyte analysis were undertaken using our routine blood counting analyser (Coulter Gen-S; Beckman Coulter, Fullerton, CA).
All laboratory examinations were performed locally according to good clinical practice. The coefficient of variation for these methods, over the range of measurement, was less than 5% as established by routine quality control procedures.
Assessment of CRP and Fbg
Routine laboratory analysis of CRP and Fbg concentration was carried out. The coefficient of variation for these methods, over the range of measurement, was less than 5% as established by routine quality control procedures.
Serum levels of proinflammatory cytokines (IL-1β, IL-6, and TNFα) and leptin.
Proinflammatory cytokines (IL-1β, IL-6, and TNFα) were detected by a “sandwich” enzyme-linked immunosorbent assay (ELISA) test (Biosource Europe SA, Nivelles, Belgium, for IL-6 and TNFα; Immunotech SA, Marseille, France for IL-1β) using monoclonal antibodies for 2 different epitopes of the cytokine molecule. The absorbance of the sample at 450 nm for IL-6 and TNFα and at 405 nm for IL-1β was measured with a spectrophotometer (Sirio; Seac, Florence, Italy). A standard curve was prepared by plotting the absorbance value of the standards versus corresponding concentrations. The concentration of the cytokine in the sample was determined by extrapolating from the standard curve. Intra-assay variations were 3% for IL-6 and 6% for TNFα and IL-1β. Interassay variations were 7% for TNFα, 8% for IL-6, and 7% for IL-1β. The results were expressed in picogram per milliliter. Serum levels of leptin were measured by a double-antibody “sandwich” ELISA test (DRG Instruments, Marburg, Germany) by using a monoclonal antibody specific for human leptin. The absorbance was measured at 450 ± 10 nm in a plate reader. The intra-assay and interassay variations were 5% and 7%, respectively. The results were expressed in nanogram per milliliter. More details of the techniques used are described in our previous reports.9,10
Assessment of blood levels of reactive oxygen species (ROS) and antioxidant enzymes glutathione peroxidase (GPx) and superoxide dismutase (SOD)
The ROSs were determined using the FORt test (Callegari, Parma, Italy), which is based on the Fenton reaction. When a 20-μL blood sample was dissolved in an acidic buffer, the hydroperoxides reacted with the transition metal ions liberated from the proteins in the acidic medium and were converted to alkoxy- and peroxy-radicals. The radical species produced by the reaction, which are directly proportional to the quantity of lipid peroxides present in the sample,17,18 interact with an additive (phenylendiamine derivative) that forms a radical molecule evaluable by spectrophotometer at 505 nm (Form CR 2000; Callegari). Results are expressed as FORT U (Fort units) whereby 1 FORT U corresponds to 0.26 mg/L H2O2.18
Erythrocyte GPx and SOD activity was measured using a commercially available kit (Ransod; Randox Lab, Crumlin, United Kingdom). The results obtained are expressed in unit per liter (U/L) of whole blood (GPx) and in unit per milliliter (U/mL) of whole blood (SOD).8
Statistical analysis
Data are represented as means plus or minus standard deviation unless otherwise stated. The significance of the difference between the mean values of patients with cancer divided according to stage of disease (stages I-II and stages III-IV) and control group was determined using 2-tailed Student t test or its nonparametric equivalent, the Mann-Whitney U test, when appropriate. Correlations between Hb and clinical (stage of disease, ECOG PS, weight, and BMI) and laboratory variables (CRP, Fbg, IL-1α, IL-6, TNFα, leptin, ROS, SOD, and GPx) were tested using 2-sided Spearman rank correlation analysis, using Bonferroni correction for multiple tests. Significant relationships were then examined by multivariate linear regression analysis against Hb (dependent variable). Results were regarded as significant if P values were .05 or less.
Results
Hb and other hematologic parameters (RBC, MCV, MCHC, reticulocyte count, serum iron, and ferritin)
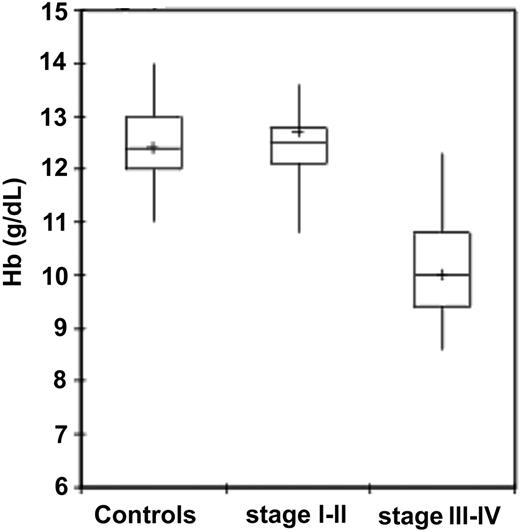
Among patients with epithelial ovarian cancer, patients at stages III to IV of disease showed significantly lower Hb levels compared with control group subjects (P < .001) and patients at stages I to II (P < .001). In patients at stages I to II of disease, in turn, Hb levels were not significantly different from control subjects (Table 2; Figure 1). As for other hematologic and iron parameters, it is to be noted that patients with stages III to IV epithelial ovarian cancer had a significantly lower RBC and reticulocyte counts, lower MCV, lower serum iron levels, and significantly higher serum ferritin levels in comparison to healthy individuals and patients at stages I to II (Table 2).
Evaluation of Hb, hematologic variables (RBCs, MCV, MCHC, absolute reticulocyte count), and iron parameters (serum iron and ferritin) in 95 control subjects and in 91 patients with epithelial ovarian cancer according to stage
Parameters . | Control subjects . | Stages I-II, n = 25 . | Stages III-IV, n = 66 . | P* . |
|---|---|---|---|---|
| Hb, g/dL | 12.4 ± 1.1 | 12.7 ± 1.1 | 10.0 ± 1.1† | < .001 |
| RBCs, 106/mL | 4.52 ± 0.3 | 4.4 ± 0.5 | 4.0 ± 0.4† | < .001 |
| MCV, fL | 87.3 ± 4.7 | 87.1 ± 6.1 | 84.7 ± 4.3† | .038 |
| MCHC, g/dL | 33.3 ± 1.5 | 33.1 ± 1.6 | 32.8 ± 2.1 | .520 |
| Reticulocytes, % | 1.35 ± 0.4 | 1.43 ± 0.4 | 0.88 ± 0.5† | < .001 |
| Reticulocytes, 103/mL | 63.6 ± 12.1 | 62.7 ± 17.9 | 39.2 ± 25.8† | < .001 |
| Serum iron, μg/dL | 93.4 ± 20.5 | 87.8 ± 33.5 | 49.3 ± 19.8† | < .001 |
| Ferritin, ng/mL | 56 ± 19 | 58 ± 32.9 | 320.4 ± 142.7† | < .001 |
Parameters . | Control subjects . | Stages I-II, n = 25 . | Stages III-IV, n = 66 . | P* . |
|---|---|---|---|---|
| Hb, g/dL | 12.4 ± 1.1 | 12.7 ± 1.1 | 10.0 ± 1.1† | < .001 |
| RBCs, 106/mL | 4.52 ± 0.3 | 4.4 ± 0.5 | 4.0 ± 0.4† | < .001 |
| MCV, fL | 87.3 ± 4.7 | 87.1 ± 6.1 | 84.7 ± 4.3† | .038 |
| MCHC, g/dL | 33.3 ± 1.5 | 33.1 ± 1.6 | 32.8 ± 2.1 | .520 |
| Reticulocytes, % | 1.35 ± 0.4 | 1.43 ± 0.4 | 0.88 ± 0.5† | < .001 |
| Reticulocytes, 103/mL | 63.6 ± 12.1 | 62.7 ± 17.9 | 39.2 ± 25.8† | < .001 |
| Serum iron, μg/dL | 93.4 ± 20.5 | 87.8 ± 33.5 | 49.3 ± 19.8† | < .001 |
| Ferritin, ng/mL | 56 ± 19 | 58 ± 32.9 | 320.4 ± 142.7† | < .001 |
Data are reported as mean ± standard deviation (SD). Hb indicates hemoglobin; RBCs, red blood cells; MCV, mean cell volume; MCHC, mean cell hemoglobin concentration.
P value was calculated with Student t test (patients with stages III-IV vs patients with stages I-II)
P < .05 in comparison to control subjects
Hemoglobin levels in 95 control subjects and 91 patients with epithelial ovarian cancer according to stage. Hemoglobin levels were significantly lower in patients with ovarian cancer than in control subjects (P < .001). Patients with stages III to IV cancer had significantly lower hemoglobin levels than patients with stages I to II (P < .001). Significance was calculated by 2-sided Student t test. The box plots in the figure represent columns of data as boxes whose extents indicate the 25th and 75th percentiles of the column. The line inside the box represents the median. + marks the value of the mean. Capped bars indicate the minimum and maximum value observed.
Hemoglobin levels in 95 control subjects and 91 patients with epithelial ovarian cancer according to stage. Hemoglobin levels were significantly lower in patients with ovarian cancer than in control subjects (P < .001). Patients with stages III to IV cancer had significantly lower hemoglobin levels than patients with stages I to II (P < .001). Significance was calculated by 2-sided Student t test. The box plots in the figure represent columns of data as boxes whose extents indicate the 25th and 75th percentiles of the column. The line inside the box represents the median. + marks the value of the mean. Capped bars indicate the minimum and maximum value observed.
CRP and Fbg in patients with epithelial ovarian cancer and control subjects
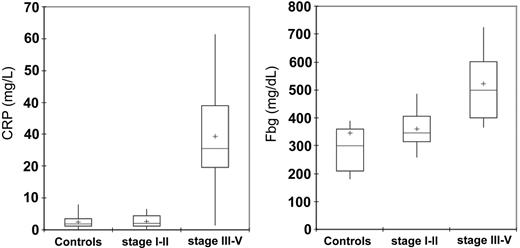
CRP and Fbg levels were significantly higher in patients with epithelial ovarian cancer at stages III to IV of disease in comparison to the control group subjects (P < .001) and patients at stages I to II of disease (P < .001). In patients at stages I to II of disease, in turn, CRP and Fbg levels were not significantly different from control subjects (Table 3; Figure 2).
Evaluation of laboratory parameters of inflammation and oxidative stress in 95 control subjects and in 91 patients with epithelial ovarian cancer according to stage
Parameters . | Control subjects . | Stages I-II, n = 25 . | Stages III-IV, n = 66 . | P* . |
|---|---|---|---|---|
| CRP, mg/L | 2.6 ± 2.0 | 2.7 ± 1.9 | 29.0 ± 15.2† | < .001 |
| Fibrinogen, mg/dL | 345.0 ± 80.5 | 355.7 ± 62.5 | 523.5 ± 132.4† | < .001 |
| IL-1β, pg/mL | 13.0 ± 5.1 | 14.0 ± 5.1 | 26.7 ± 19.4† | .007 |
| IL-6, pg/mL | 1 ± 2.5 | 8.7 ± 5.6§ | 32.8 ± 23.2† | < .001 |
| TNFα, pg/mL | 19 ± 6.7 | 21.6 ± 7.7 | 39.4 ± 21.4† | .003 |
| Leptin, ng/mL | 19 ± 16.1 | 14.6 ± 9.8 | 7.1 ± 6.3† | .009 |
| ROS, Fort U | 197.5 ± 56.7 | 270.7 ± 39.5§ | 431.4 ± 69.5† | < .001 |
| GPx, U/L | 10 344.3 ± 2280.3 | 9984 ± 1563.0 | 6560.6 ± 2226.6† | < .001 |
| SOD, U/mL | 89.5 ± 18.4 | 80.6 ± 29.4 | 69.3 ± 32.9† | .028 |
Parameters . | Control subjects . | Stages I-II, n = 25 . | Stages III-IV, n = 66 . | P* . |
|---|---|---|---|---|
| CRP, mg/L | 2.6 ± 2.0 | 2.7 ± 1.9 | 29.0 ± 15.2† | < .001 |
| Fibrinogen, mg/dL | 345.0 ± 80.5 | 355.7 ± 62.5 | 523.5 ± 132.4† | < .001 |
| IL-1β, pg/mL | 13.0 ± 5.1 | 14.0 ± 5.1 | 26.7 ± 19.4† | .007 |
| IL-6, pg/mL | 1 ± 2.5 | 8.7 ± 5.6§ | 32.8 ± 23.2† | < .001 |
| TNFα, pg/mL | 19 ± 6.7 | 21.6 ± 7.7 | 39.4 ± 21.4† | .003 |
| Leptin, ng/mL | 19 ± 16.1 | 14.6 ± 9.8 | 7.1 ± 6.3† | .009 |
| ROS, Fort U | 197.5 ± 56.7 | 270.7 ± 39.5§ | 431.4 ± 69.5† | < .001 |
| GPx, U/L | 10 344.3 ± 2280.3 | 9984 ± 1563.0 | 6560.6 ± 2226.6† | < .001 |
| SOD, U/mL | 89.5 ± 18.4 | 80.6 ± 29.4 | 69.3 ± 32.9† | .028 |
Data are reported as mean ± standard deviation (SD). CRP indicates C-reactive protein; IL, interleukin; TNFα, tumor necrosis factor α; ROS, reactive oxygen species; GPx, glutathione peroxidase; SOD, superoxide dismutase.
P value was calculated with Student t test (patients with stages III-IV vs patients with stages I-II)
P < .05 in comparison to control subjects
Serum levels of proinflammatory cytokines (IL-1β, IL-6, and TNFα) and leptin in patients with epithelial ovarian cancer and control subjects
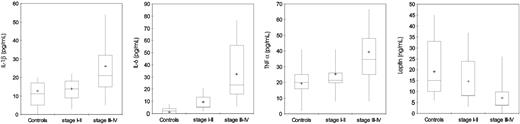
Serum levels of IL-1β and TNFα were significantly higher in patients with advanced epithelial ovarian cancer (stages III-IV) compared with control subjects (P < .001) and patients with stages I to II disease (P = .007, P < .001, and P = .003, respectively). Patients at stages I to II of disease, in turn, did not show significantly different levels of IL-1β and TNFα compared with control subjects. Levels of IL-6 were significantly higher in patients with epithelial ovarian cancer both in stages III to IV and stages I to II compared with control subjects (P < .001, for both) (Table 3; Figure 3). Serum leptin levels were significantly lower in patients with stages III to IV epithelial ovarian cancer in comparison to control subjects (P < .001) and patients in stages I to II (P < .009). In patients with epithelial ovarian cancer at stages I to II of disease, in turn, leptin levels were not significantly different from control subjects (Table 3; Figure 3).
Serum levels of CRP and Fbg in 95 control subjects and 91 patients with epithelial ovarian cancer according to stage. Serum levels of CRP and Fbg were significantly lower in patients with cancer than in control subjects (P < .001 and P = .04, respectively). Moreover, patients with stages III to IV ovarian cancer had significantly higher CRP and Fbg levels than did patients with stages I to II (P < .001, for both). Significance was calculated by 2-sided Student t test. The box plots in the figure represent columns of data as boxes whose extents indicate the 25th and 75th percentiles of the column. The line inside the box represents the median. + marks the value of the mean. Capped bars indicate the minimum and maximum value observed.
Serum levels of CRP and Fbg in 95 control subjects and 91 patients with epithelial ovarian cancer according to stage. Serum levels of CRP and Fbg were significantly lower in patients with cancer than in control subjects (P < .001 and P = .04, respectively). Moreover, patients with stages III to IV ovarian cancer had significantly higher CRP and Fbg levels than did patients with stages I to II (P < .001, for both). Significance was calculated by 2-sided Student t test. The box plots in the figure represent columns of data as boxes whose extents indicate the 25th and 75th percentiles of the column. The line inside the box represents the median. + marks the value of the mean. Capped bars indicate the minimum and maximum value observed.
Serum levels of IL-1β, IL-6, TNFα, and leptin in 95 control subjects and 91 patients with epithelial ovarian cancer according to stage. Patients with ovarian cancer showed significantly higher (P < .001) serum levels of IL-1β, IL-6, and TNFα and lower leptin in comparison with control subjects. Moreover, patients with stages III to IV cancer showed higher levels of IL-1β, IL-6, and TNFα and lower levels of leptin in comparison to patients with stages I to II. Significance was calculated by 2-sided Student t test, while for leptin the significance was calculated using the Mann-Whitney U test. The box plots in the figure represent columns of data as boxes whose extents indicate the 25th and 75th percentiles of the column. The line inside the box represents the median. + marks the value of the mean. Capped bars indicate the minimum and maximum value observed.
Serum levels of IL-1β, IL-6, TNFα, and leptin in 95 control subjects and 91 patients with epithelial ovarian cancer according to stage. Patients with ovarian cancer showed significantly higher (P < .001) serum levels of IL-1β, IL-6, and TNFα and lower leptin in comparison with control subjects. Moreover, patients with stages III to IV cancer showed higher levels of IL-1β, IL-6, and TNFα and lower levels of leptin in comparison to patients with stages I to II. Significance was calculated by 2-sided Student t test, while for leptin the significance was calculated using the Mann-Whitney U test. The box plots in the figure represent columns of data as boxes whose extents indicate the 25th and 75th percentiles of the column. The line inside the box represents the median. + marks the value of the mean. Capped bars indicate the minimum and maximum value observed.
Blood levels of ROS and antioxidant enzymes GPx and SOD in patients with epithelial ovarian cancer and in control subjects
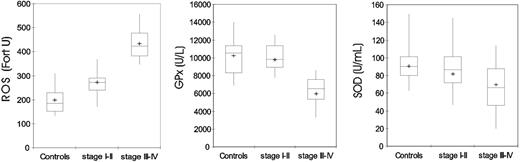
Blood levels of ROS were significantly higher in patients with epithelial ovarian cancer (both stages III-IV and stages I-II) compared with control subjects (P < .001). Blood levels of GPx and SOD were significantly lower in patients with stages III to IV epithelial ovarian cancer compared with control subjects (P < .001, for both) and patients in stages I to II (P < .001 for GPx and P = .028 for SOD). In patients with stages I to II of disease, GPx and SOD were not significantly different from control subjects (Table 3; Figure 4).
Correlation between hemoglobin, clinical parameters (stage of disease and ECOG PS) and serum levels of proinflammatory cytokines, CRP, Fbg, ROS, and antioxidant enzymes (SOD and GPx)
A significant negative relationship was found between Hb levels and stage of disease (r = -0.84, P < .001) and ECOG PS (r = -0.82, P < .001). A significant positive relationship was found between Hb levels and weight (r = 0.36, P = .022) and BMI (r = 0.48, P = .001). As for laboratory variables, a significant negative correlation was found between Hb levels and CRP (r = -0.73, P = < .001), Fbg (r = -0.67, P < .001), IL-1β (r = -0.50, P = .001), IL-6 (r = -0.78, P < .001), TNFα (r = -0.52, P = .001), and ROS (r = -0.73, P < .001) levels. A significant positive correlation was found between Hb levels and serum levels of leptin (r = 0.455, P = .001) and blood levels of GPx (r = 0.62, P < .001). SOD levels did not show a significant correlation with Hb levels (r = 0.19, P = .245) (Table 4). Significant relationships were further included in multivariate regression analysis. With regard to Hb (dependent variable), the evaluation revealed that only stage (coefficient = -0.5906, P = .035) and IL-6 levels (coefficient = -0.0230, P = 0.046) were independent factors predicting Hb levels (Table 5). Moreover, we also tested whether IL-6 was a predictive variable of the other proinflammatory markers: single regression analysis showed that IL-6 was significantly positively correlated with IL-1β (P = .020), CRP (P < .001), Fbg (P = .001), and ROS (P < .001) (Figure 5).
Correlation of Hb levels with clinical parameters (stage, weight, BMI) and markers of chronic inflammation and oxidative stress in 91 patients with epithelial ovarian cancer
. | Spearman r . | P . |
|---|---|---|
| Clinical parameters | ||
| Stage | -0.84 | < .001 |
| ECOG PS | -0.82 | < .001 |
| Weight | 0.36 | .022 |
| BMI, weight/height2 | 0.48 | .001 |
| Laboratory parameters | ||
| CRP | -0.73 | < .001 |
| Fibrinogen | -0.67 | < .001 |
| IL-1β | -0.50 | .001 |
| IL-6 | -0.78 | < .001 |
| TNFα | -0.52 | .001 |
| Leptin | 0.55 | .001 |
| ROS | -0.73 | < .001 |
| GPx | 0.62 | < .001 |
| SOD | 0.19 | .245 |
. | Spearman r . | P . |
|---|---|---|
| Clinical parameters | ||
| Stage | -0.84 | < .001 |
| ECOG PS | -0.82 | < .001 |
| Weight | 0.36 | .022 |
| BMI, weight/height2 | 0.48 | .001 |
| Laboratory parameters | ||
| CRP | -0.73 | < .001 |
| Fibrinogen | -0.67 | < .001 |
| IL-1β | -0.50 | .001 |
| IL-6 | -0.78 | < .001 |
| TNFα | -0.52 | .001 |
| Leptin | 0.55 | .001 |
| ROS | -0.73 | < .001 |
| GPx | 0.62 | < .001 |
| SOD | 0.19 | .245 |
ECOG PS indicates Eastern Cooperative Oncology Group Performance Status; BMI, body mass index; CRP, C-reactive protein; IL, interleukin; TNFα, tumor necrosis factor α; ROS, reactive oxygen species; GPx; glutathione peroxidase; SOD, superoxide dismutase.
Multiple regression analysis of stage and laboratory variables with regard to Hb levels in 91 epithelial ovarian cancer patients
Covariate parameters . | Coefficient . | SE . | 95% Confidence Interval . | P . |
|---|---|---|---|---|
| Stage | -0.5906 | 0.2680 | -1.1372 to -0.0440 | .035 |
| Weight | -0.0326 | 0.0406 | -0.1150 to 0.0499 | .427 |
| BMI | 0.1125 | 0.1102 | -0.1112 to 0.3361 | .314 |
| CRP | 0.0029 | 0.0203 | -0.0384 to 0.0442 | .887 |
| Fbg | -0.0013 | 0.0020 | -0.0055 to 0.0029 | .528 |
| IL-1β | -0.0096 | 0.0131 | -0.0363 to 0.0171 | .468 |
| IL-6 | -0.0230 | 0.0111 | -0.0457 to -0.0004 | .046 |
| TNFα | -0.0122 | 0.0106 | -0.0337 to 0.0093 | .255 |
| ROS | -0.0014 | 0.0031 | -0.0078 to 0.0050 | .659 |
| GPx | 0.0000 | 0.0001 | -0.0002 to 0.0001 | .629 |
Covariate parameters . | Coefficient . | SE . | 95% Confidence Interval . | P . |
|---|---|---|---|---|
| Stage | -0.5906 | 0.2680 | -1.1372 to -0.0440 | .035 |
| Weight | -0.0326 | 0.0406 | -0.1150 to 0.0499 | .427 |
| BMI | 0.1125 | 0.1102 | -0.1112 to 0.3361 | .314 |
| CRP | 0.0029 | 0.0203 | -0.0384 to 0.0442 | .887 |
| Fbg | -0.0013 | 0.0020 | -0.0055 to 0.0029 | .528 |
| IL-1β | -0.0096 | 0.0131 | -0.0363 to 0.0171 | .468 |
| IL-6 | -0.0230 | 0.0111 | -0.0457 to -0.0004 | .046 |
| TNFα | -0.0122 | 0.0106 | -0.0337 to 0.0093 | .255 |
| ROS | -0.0014 | 0.0031 | -0.0078 to 0.0050 | .659 |
| GPx | 0.0000 | 0.0001 | -0.0002 to 0.0001 | .629 |
The dependent variable (Hb) in the regression equation, the number of patients observed (91), the number of terms in the regression equation (10), the coefficient of determination (R2 = 0.75) and P value (< .001) are used. The results showed that Hb is predicted only by stage and IL-6 levels. In contrast to these parameters C-reactive protein (CRP), fibrinogen (Fbg), leptin, TNFα, reactive oxygen species (ROS), and glutathione peroxidase (GPx) showed a significant correlation with Hb at single regression analysis but failed to be independent factors for Hb levels in multiple regression analysis. Text in italics indicates that the P value is statistically significant (P < 0.5).
BMI indicates body mass index; IL, interleukin; TNF, tumor necrosis factor; ROS, reactive oxygen species; SOD, superoxide dismutase.
Blood levels of ROS, GPx, and SOD in 95 control subjects and 91 patients with epithelial ovarian cancer according to stage. ROS levels were significantly higher (P < .001), while GPx and SOD activities were significantly lower in patients with cancer than in control subjects (P < .001). P was calculated by 2-sided Student t test. The box plots in the figure represent columns of data as boxes whose extents indicate the 25th and 75th percentiles of the column. The line inside the box represents the median. + marks the value of the mean. Capped bars indicate the minimum and maximum value observed.
Blood levels of ROS, GPx, and SOD in 95 control subjects and 91 patients with epithelial ovarian cancer according to stage. ROS levels were significantly higher (P < .001), while GPx and SOD activities were significantly lower in patients with cancer than in control subjects (P < .001). P was calculated by 2-sided Student t test. The box plots in the figure represent columns of data as boxes whose extents indicate the 25th and 75th percentiles of the column. The line inside the box represents the median. + marks the value of the mean. Capped bars indicate the minimum and maximum value observed.
Discussion
The study was designed to determine whether those factors that have been demonstrated to play a critical role in the onset of cachexia, weight loss, and impairment of performance status are also involved in the development of anemia. To test this hypothesis, 91 previously untreated patients with epithelial ovarian cancer were evaluated. As expected, Hb levels were lower in patients than in appropriately matched control subjects. Furthermore, as previously shown by Obermair et al,1 Hb concentrations were inversely related to stage of disease and severity of chronic inflammatory status. In our study, advanced epithelial ovarian cancer (stages III-IV) showed anemia similar to anemia of inflammation (also referred to as anemia of chronic disease), which is characterized by normocytic or microcytic iron-refractory anemia, low serum iron, and preserved bone marrow iron.19 The lowest Hb levels associated with the highest concentrations of markers of inflammation, such as proinflammatory cytokines (IL-6, IL-1β, TNFα), CRP, and Fbg, and with the lowest leptin levels. Statistical analysis confirmed that Hb inversely correlated with stage and ECOG PS, proinflammatory cytokines (IL-6, IL-1β, TNFα), CRP, Fbg, and ROS but positively correlated with leptin and GPx. By multivariate regression analysis, only stage of disease and IL-6 levels were independent factors in determining Hb levels.
Van der Zee et al20 demonstrated that higher levels of interleukin-6 in cystic fluids from patients with malignant versus benign ovarian tumors correlate with decreased hemoglobin levels and increased platelet counts.
A central role for IL-6 in the induction of both CRA and the anemia of inflammatory disease has been recently demonstrated. A specific role for IL-6 in the pathogenesis of anemia has been suggested by Ershler21 who has found an increased level of IL-6 with advanced age, presumably because of an age-acquired dysregulation, and a corresponding increased prevalence of anemia. Moreover, several researchers22,23 have demonstrated that IL-6 is both necessary and sufficient for the induction of hepcidin, an iron regulatory hormone responsible for inflammation-induced iron disutilization resulting in the anemia associated with acute and chronic infections,24,25 chronic kidney disease,26 and presumably neoplastic disease.27
Single regression analysis between IL-6 and markers of chronic inflammation and oxidative stress. IL-6 was significantly correlated to (A) IL-1β (P = .020), (B) C-reactive protein (P < .001), (C) reactive oxygen species (ROS; P < .001), and (D) fibrinogen (Fbg; P = .001). The central straight line corresponds to the best-fit linear regression line. The two curved lines surrounding the best-fit line define the 95% confidence interval of the regression line. The graphs also show the prediction interval (the curves defining the prediction interval are further from the regression line than the confidence lines).
Single regression analysis between IL-6 and markers of chronic inflammation and oxidative stress. IL-6 was significantly correlated to (A) IL-1β (P = .020), (B) C-reactive protein (P < .001), (C) reactive oxygen species (ROS; P < .001), and (D) fibrinogen (Fbg; P = .001). The central straight line corresponds to the best-fit linear regression line. The two curved lines surrounding the best-fit line define the 95% confidence interval of the regression line. The graphs also show the prediction interval (the curves defining the prediction interval are further from the regression line than the confidence lines).
The pattern of IL-6 and Hb found in our study in patients with ovarian cancer needs to be further evaluated for other types of cancer. In fact, it could be linked with a specific production of IL-6 by ovarian cancer cells and not a marker of inflammation. Higher serum levels of IL-6 have been found in patients with ovarian cancer than in patients with other malignancies,28 and levels have been shown to correlate with extent of disease29 and unfavorable clinical outcome.30 However, in our previously published studies, we demonstrated that tumor-associated mononuclear cells (lymphocytes and monocytes) from neoplastic effusions and peripheral blood were able to produce high amounts of IL-6 and IL-211,31 and that increased IL-6 levels were associated with CRP and aspecific/ineffective immune response.12 As yet it is not clear whether increased IL-6 levels in patients with advanced ovarian cancer are produced by the tumor itself or mainly by host tissues.
Of note, in the current study we demonstrated a significant positive correlation between IL-6 and other markers of inflammation and oxidative stress (IL-1β, CRP, Fbg, and ROS). Thus, high serum levels of IL-6 may be considered an indicator of the inflammatory and pro-oxidative status of patients with epithelial ovarian cancer.
The study did not demonstrate the mechanism through which the high levels of inflammatory mediators could induce CRA. However, several studies, in addition to those cited earlier, showed that proinflammatory cytokines blunt EPO response to anemia and impair erythroid colony formation in response to EPO.32-34 Additionally, proinflammatory cytokines IL-1, IL-6, TNFα, and the acute-phase proteins impair iron metabolism, inhibiting the reticuloendothelial iron stores with low iron circulating levels.35-37 Disorder in iron reutilization characterizes also CRA.38
The presence of proinflammatory cytokines in patients with neoplasms has been associated with increased production of ROS (H2O2 and
The lower leptin levels found in our patients with advanced ovarian cancer may be the consequence of cancer-related nutritional inadequacy and impaired energy metabolism or the direct result of high levels of IL-6 or the other cytokines.9,10 Takeda et al41 hypothesized that nutritional status, probably through leptin action, may affect erythropoiesis and demonstrated that BMI and leptin were inversely correlated with the recombinant human EPO (rHuEPO) dose required in patients receiving hemodialysis. Indeed, in vitro studies have suggested that leptin plays a role in enhancing erythropoiesis,42 but, certainly, this hypothesis needs more definitive analysis.
The results we have presented suggest that anemia in patients with epithelial ovarian cancer is, at least in part, the consequence of cancer-related chronic inflammation. Cancer-related anemia must be recognized as a constitutional feature of patients with advanced neoplasms and not necessarily as just a consequence of antineoplastic treatments. Indeed, it has been widely demonstrated that CRA is associated with poor response to treatment and decreased survival,43 and with a decline in energy and activity levels, quality of life, and cognitive functions.44 An increased understanding of the pathogenesis of CRA may help identify the most appropriate treatment strategies.22,45
Prepublished online as Blood First Edition Paper, March 17, 2005; DOI 10.1182/blood-2005-01-0160.
Supported by Associazione sarda per la ricerca in oncologia ginecologica ONLUS.
A.M. designed the research, performed the research, analyzed the data, and wrote the paper; C.M. designed the research, performed the research, contributed vital new reagents or analytical tools, analyzed the data, and wrote the paper; D.M., M.C.M., M.R.L., and G.G. performed the research, contributed to patient enrollment and assessment, and collected the data; R.S. performed the research and contributed to the analytical tools; and G.B.M. and G.M. contributed to the design of the research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal