Abstract
The pathologische anatomie Leiden-endothelium (PAL-E) antibody has been used for almost 20 years as a specific marker for vascular endothelial cells. Due to the fact that this antibody works only in very limited applications, the molecular identity of PAL-E has remained unknown. In this work, we demonstrate by double stainings, cross-immunoprecipitations, and transfectants that the PAL-E antigen is identical with a protein designated PV-1 (plasmalemmal vesicle 1) or FELS (fenestrated endothelial-linked structure protein) and is not vimentin, as reported earlier. As the expression of this molecule is by no means restricted to fenestrated endothelium, we suggest the use of the name PLVAP for this protein. Molecular identification of PLVAP should help in the production of new tools for the identification of vascular as opposed to lymphatic endothelium and to elucidate the function of this protein.
Introduction
Several antigens including factor VIII, CD31, CD36, ulex europaeus lectin, and pathologische anatomie Leiden-endothelium (PAL-E) have been used as endothelial cell markers in a vast number of publications. However, none of these markers is perfect, because they either do not stain all types of endothelia or are not specific for vasculature. PAL-E has been considered a good marker because it discriminates blood vessel endothelium from lymphatic endothelium and does not stain other cell types.1-3 Despite the relatively wide use of PAL-E as an endothelial marker, its molecular identity has remained an enigma, most likely due to the limited applicability of the antibody against the PAL-E molecule. However, a recent report suggests that the PAL-E antigen is a secreted form of vimentin.4 The molecule defined by PAL-E is widely expressed on vasculature in different organs except in the brain. However, its expression is induced in brain tumors simultaneously with the loss of the blood-brain barrier.5 Expression of PAL-E antigen is also increased on endocardium of rejected human cardiac allografts.6
Plasmalemmal vesicle 1 (PV-1) has been described as a glycoprotein associated to plasmalemmal vesicles (caveolae). It is a 60-type II transmembrane protein that tends to form homodimers. Its cellular localization in the rat has been carefully analyzed both at light and electron microscopic levels.7,8 However, the function of PV-1 has remained unknown. Expression of rat PV-1 is developmentally regulated in testis and it binds heparin.9 The protein sequences of rat and mouse PV-1 have phosphorylation site(s) for casein kinase II, but this site is lacking in the human sequence.10 The corresponding human sequence does not bear significant homology to any other known proteins, and, therefore, the sequence information has not helped in predicting the function of this molecule.10
Our original aim was to identify new endothelial molecules involved in leukocyte trafficking. For this purpose, we raised monoclonal antibodies (mAbs) against small isolated vessels from the hilus of human lymph nodes. The expression pattern of one antibody showed striking specificity for endothelial cells, and, therefore, the antigen recognized by this antibody was selected for further characterization. Tryptic peptides and subsequent analyses of its cDNA showed homology of this antigen to rat PV-1 cDNA and a human cDNA designated fenestrated endothelial-linked structure protein (FELS). On the other hand, the similarities in the staining patterns between our antibody and PAL-E led to the present studies elucidating the identity of the molecule recognized by PAL-E.
Materials and methods
Production of the monoclonal antibody
Balb/C mice were immunized by injecting into the footpads a suspension containing isolated hilar vessels from human lymph nodes and Freund incomplete adjuvant in phosphate-buffered saline (PBS) 3 times with one-week intervals between injections. Three days after the last immunization, the popliteal lymph nodes were collected and the isolated lymphocytes were fused with SP2/0 myeloma cells. The hybridoma supernatants were screened by immunohistochemistry using frozen sections of human tonsils. Hybridoma 174/2 was selected for further analysis because of its highly specific endothelial staining pattern.
Peptide and cDNA analyses
The molecule recognized by 174/2 antibody from human lymph node lysate was purified with CnBr-Sepharose 4B (Pharmacia, Uppsala, Sweden) columns coupled to 174/2 antibody as described,11 and the eluted material was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis and silver staining. We excised the specific band and reduced, alkylated, and digested it with trypsin overnight at +37°C. The peptides were analyzed with PerSeptive Biosystems Voyager DE-PRO mass spectrometer (Applied Biosystems, Foster City, CA), and the databases were searched using MS-Fit algorithm (http://prospector.ucsf.edu/ucsfhtml4.0/msfit.htm) of the University of California San Francisco (UCSF) mass spectrometry facility.
Based on the sequence data available from GenBank submissions AF326591 (FELS) and AF154831 (rat PV-1), we designed specific primers in order to produce the full-length cDNA by reverse transcriptase–polymerase chain reaction (RT-PCR).
RNA from human lymph nodes was extracted using the Ultraspec RNA reagent (Biotecx Laboratories, Houston, TX). Total RNA (1 μg) was reverse-transcribed with Expand Reverse Transcriptase (Roche Molecular Biochemicals, Mannheim, Germany) as suggested by the manufacturer. The reverse-transcribed RNA (2 μL) was used in RT-PCR reactions using the Expand Long Template PCR System (Roche Molecular Biochemicals) according to the instructions of the supplier. The primers used were as follows: forward primer, 5′-CACGCGTCGGGTGGTGAGCAG-3′ and reverse primer, 5′-GTGCCACCAACATTTCAACGG-3′. The PCR products were run on 1% agarose gels; the bands with the correct size of approximately 2260 base pairs (bp) were collected and cloned into the pGEM-T Easy vector (Promega, Madison, WI) for sequencing. The verified full-length cDNA was further cloned into the expression vector pcDNA3.1 (Clontech, BD Biosciences, Palo Alto, CA) for transfection studies.
Immunostainings
Immunofluorescence and immunohistochemical stainings were made on 6-μm–thick acetone-fixed frozen sections of lymph nodes, aorta, large vein, tonsil, kidney, liver, brain, intestine, and appendix. Commercial PAL-E antibody (mouse immunoglobulin G2a [IgG2a]; dilution ratio, 1:100; Abcam, Cambridge, United Kingdom) and 174/2 antibody (mouse IgG1, 20 μg/mL) were used as primary antibodies in immunofluorescence stainings. In addition, fluorescein isothiocyanate (FITC)–conjugated anti–factor VIII antibody (dilution ratio, 1:500; INCSTAR, Saluggia, Italy) was used as a marker for vessels, and monoclonal anti–mannose receptor antibody, 3-155,11 and polyclonal (rabbit) anti–human Lyve-1 (Research Diagnostics, Basel, Switzerland) served as markers for lymphatics. Negative class-matched control antibodies were 3G6 (mouse [IgG1] anti–chicken T cells), 11.7-3 (mouse [IgG2a] anti–chicken IgG), and normal rabbit serum. Peroxidase-conjugated rabbit anti–mouse immunoglobulins (dilution ratio, 1:100; DAKO, Glostrup, Denmark) were used as a secondary antibody in immunohistochemical stainings. In immunofluorescence stainings, either FITC- or R-phycoerythrin (R-PE)–conjugated goat anti–mouse IgG1 and goat anti–mouse IgG2a (dilution rate, 1:100; Southern Biotechnology Associates, Mississauga, ON) were used as secondary antibodies for 174/2 and PAL-E, and FITC–anti–rabbit Ig (Sigma, St Louis, MO) was used for Lyve-1.
For fluorescence-activated cell sorter (FACS) analyses, detached Chinese hamster ovary (CHO) cells transfected (electroporation: 300 V, 975 μF) with human PV-1/FELS cDNA or mock-transfected cells were incubated with PAL-E antibody (1:100; Abcam), antivimentin antibody (V9, 5 μg/mL; Chemicon, Temecula, CA), or a negative control antibody for 15 minutes followed by a second-stage antibody, FITC-conjugated anti–mouse Ig (1:100; Sigma) for 15 minutes. Per sample, 10 000 cells were analyzed using FACS Calibur (Becton Dickinson, Heidelberg, Germany) and CellQuest software.
For confocal microscopic analyses, CHO cells transfected with human PV-1/FELS cDNA or mock-transfected cells were grown on glass coverslips overnight. Cells were fixed with cold acetone. For double stainings, the coverslips were sequentially incubated with 174/2 (20 μg/mL), followed by FITC-conjugated anti–mouse IgG1. The coverslips were then incubated with second mAb to PAL-E (1:100; Abcam) followed by PE-conjugated anti–mouse IgG2a. Control stainings included irrelevant primary antibodies of the same isotype. Confocal images (Figure 2G-K) were taken with a Zeiss 510 META confocal microscope equipped with a Plan-Apochromat 63×/1.4 oil-immersion DIC objective. Images were analyzed with LSM 5 Image browser software (Carl Zeiss, Heidelberg, Germany). All other images in Figures 1, 2, 4, and 5 were taken with an Olympus BX60 microscope equipped with 10×/0.30 Ph1, 20×/0.50 Ph1, or 40×/0.75 Ph2 objective lenses, and were processed with AnalySIS software (Olympus, Tokyo, Japan).
Cross-immunoprecipitations
Proteins were immunoaffinity depleted from nonidet P-40 (NP-40) lysates of CHO cells transfected with PV-1/FELS cDNA, lymphocyte-depleted tonsil specimens, or human dermal microvascular cell line cells, HMEC-1 (lysis buffer: 150 mM NaCl, 10 mM Tris [tris(hydroxymethyl)aminomethane] base, pH 7.2, 1.5 mM MgCl2, 1% NP-40, 1% aprotinin, and 1 mM phenylmethyl sulfonyl fluoride) with protein A–rabbit anti–mouse Ig beads coupled to PAL-E, 174/2, antivimentin antibody (V9), or a negative control antibody, 3G6. Aliquots of the depleted lysates (and a nondepleted lysate as a control) were mixed with an equal volume of Laemmli sample buffer without reduction and heated for 20 minutes at 37°C. These mild conditions were needed to maintain the reactivity of PAL-E in immunoblotting. Thereafter, they were loaded on 5% to 12.5% SDS-PAGE gels. The resolved proteins were transferred onto nitrocellulose sheets (Hybond-ECL; Amersham, Freiburg, Germany) and probed with V9, PAL-E, 174/2, or 3G6 using an enhanced chemiluminescence detection kit for Western blotting (Amersham) according to the manufacturer's recommendations.
Results
Mice were immunized with isolated human vessels to produce anti–endothelial-cell antibodies. When the hybridoma supernatants were in their first screen on human tonsils, 174/2 antibody showed exceptional specificity toward endothelial cells. 174/2 stained small- and medium-sized vessels on both the venous and arterial side. This endothelial specificity held true in more extensive analyses involving peripheral lymph nodes, tonsil, skin, intestine, heart, kidney, appendix, and liver. In contrast, normal brains were virtually 174/2 negative (data not shown).
174/2 antibody worked well in immunoprecipitations and immunoblotting, and we purified enough protein for protein sequencing. We obtained 10 peptides, the sequences of which matched to the human FELS protein sequence in the data bank and covered 10% of the protein (shown in Table S1 and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). They did not match the vimentin sequence (see below). Based on the sequence of FELS, we designed primers and produced a full-length cDNA from human lymph node mRNA by RT-PCR. Transfections and subsequent stainings of the host cells clearly showed that 174/2 antibody recognizes PV-1/FELS protein.
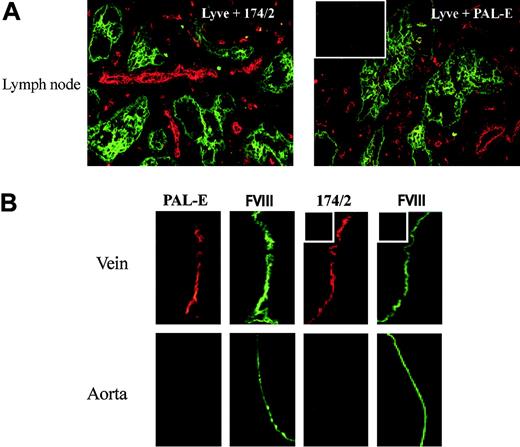
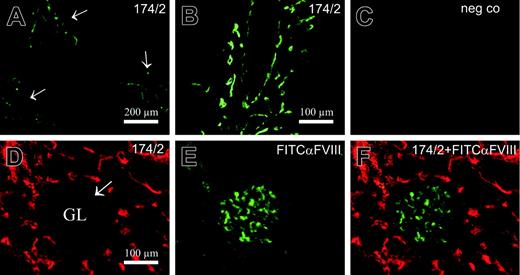
As very limited data are available on the expression pattern of PV-1 (or FELS) in different human tissues, we performed double immunofluorescence stainings with 174/2 and known markers for blood vessels and lymphatic vessels on frozen sections of various organs (kidney, lymph nodes, tonsil, and intestine) and compared the staining patterns with those of PAL-E. 174/2 and PAL-E positively stained blood vessels but lymphatics remained negative. Lymph node stainings are shown as examples in Figure 1A. Like PAL-E, 174/2 stained endothelium in large veins, while endothelial cells in the aorta remained negative (Figure 1B). When 174/2 and PAL-E antibodies were used together for double stainings, the staining patterns were identical. Peripheral lymph node and kidney stainings are shown as examples in Figure 2A-F. To further evaluate whether 174/2 and PAL-E recognize the same antigen, 2 different host cells were transfected for immunostainings. Both CHO and HEK 293 cells transfected with PV-1/FELS cDNA but not cells that were mock transfected were recognized by 174/2 and PAL-E. As an example, confocal images are shown from CHO cells transfected with the cDNA encoding PV-1/FELS (Figure 2G-J). The faint nuclear-looking staining with 174/2 (also seen with PAL-E when a higher detection gain was used) was further analyzed with Z stack imaging. The results show that the signal comes from the cytoplasm above the nucleus, and no intranuclear localization of PV-1 can be seen (Figure 2K).
Staining patterns of 174/2 and PAL-E. (A) Lymph node sections were double stained with an antibody to Lyve-1 (green) recognizing lymphatics in the tissue and 174/2 (red) or PAL-E (red) as indicated in the figure. (B) Vein and aorta specimens were double stained with FITC–anti–factor VIII (FVIII, green) to identify the vascular endothelial cells and 174/2 (red) or PAL-E (red) as indicated in the figure. The insets show stainings with negative control antibodies. Original magnification ×200 (A); ×400 (D).
Staining patterns of 174/2 and PAL-E. (A) Lymph node sections were double stained with an antibody to Lyve-1 (green) recognizing lymphatics in the tissue and 174/2 (red) or PAL-E (red) as indicated in the figure. (B) Vein and aorta specimens were double stained with FITC–anti–factor VIII (FVIII, green) to identify the vascular endothelial cells and 174/2 (red) or PAL-E (red) as indicated in the figure. The insets show stainings with negative control antibodies. Original magnification ×200 (A); ×400 (D).
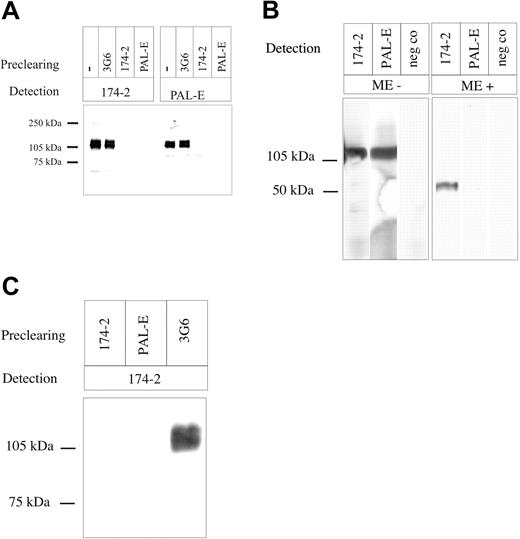
To formally confirm that 174/2 recognizes the same molecule as PAL-E, cross-immunoprecipitations were made from the CHO transfectants expressing PV-1/FELS. PAL-E was able to deplete practically all 120-kDa protein (homodimer) detected by 174/2, whereas after depletion with 174/2 a very faint, lower molecular weight form of 85 kDa was still recognized by PAL-E (Figure 3A). Most likely, this lower molecular weight form represents a degradation product of the molecule that does not bear the epitope recognized by 174/2. To confirm that PAL-E and 174/2 recognize the same antigen also on endothelium in tissues, we first performed immunoblotting from tonsil lysate. Both 174/2 and PAL-E recognized a band of the same size in nonreduced sample, whereas PAL-E did not show any reactivity when the sample was reduced (Figure 3B). We further precleared lymphocyte-depleted tonsil tissue with beads coupled to 174/2, PAL-E, or negative control antibody (3G6). 174/2 and PAL-E (but not the negative control antibody) removed 174/2 reactivity from the lysate when analyzed by immunoblotting (Figure 3C).
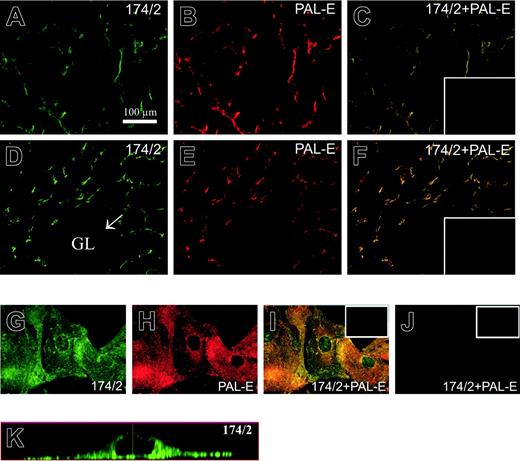
174/2 and PAL-E recognize the same molecule. Immunofluorescence stainings of lymph node (A-C), kidney (D-F), and PV-1/FELS cDNA-transfected CHO cells (G-K). The tissues and cells were stained with 174/2 (green) and PAL-E (red), and merged images are shown in panels C, F, and I. The mock-transfected CHO cells stained with 174/2 and anti–PAL-E mAbs are shown in panel J. Orthogonal sections of a Z stack were made from PV-1–transfected CHO cells stained with 174/2 antibody (K). The representative negative controls are shown in the insets.
174/2 and PAL-E recognize the same molecule. Immunofluorescence stainings of lymph node (A-C), kidney (D-F), and PV-1/FELS cDNA-transfected CHO cells (G-K). The tissues and cells were stained with 174/2 (green) and PAL-E (red), and merged images are shown in panels C, F, and I. The mock-transfected CHO cells stained with 174/2 and anti–PAL-E mAbs are shown in panel J. Orthogonal sections of a Z stack were made from PV-1–transfected CHO cells stained with 174/2 antibody (K). The representative negative controls are shown in the insets.
Cross-precipitations with 174/2 and PAL-E. Lysates of CHO cells transfected with (A) PV-1/FELS cDNA, (B) tonsil, or (C) lymphocyte-depleted tonsil tissue were precleared or not precleared with the indicated antibodies; thereafter, the samples were run on SDS-PAGE and blotted to nitrocellulose membranes. Detection was performed either with 174/2 or PAL-E. neg co indicates negative control. Molecular weight markers are indicated on the left. ME indicates mercaptoethanol.
Cross-precipitations with 174/2 and PAL-E. Lysates of CHO cells transfected with (A) PV-1/FELS cDNA, (B) tonsil, or (C) lymphocyte-depleted tonsil tissue were precleared or not precleared with the indicated antibodies; thereafter, the samples were run on SDS-PAGE and blotted to nitrocellulose membranes. Detection was performed either with 174/2 or PAL-E. neg co indicates negative control. Molecular weight markers are indicated on the left. ME indicates mercaptoethanol.
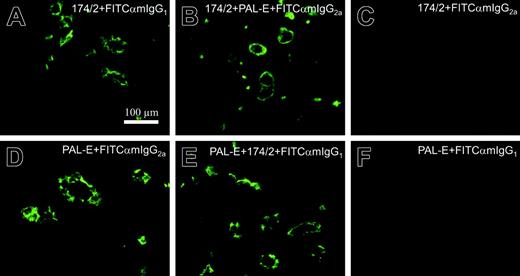
By performing competitive stainings, we also tested whether PAL-E and 174/2 recognize the same epitope. Neither one of the antibodies could inhibit the signal of the other one, indicating that the epitopes recognized by these antibodies are distinct (Figure 4).
Although in a GenBank entry the molecule recognized by 174/2 was found by the name “fenestrated endothelial-linked structure protein (FELS),” it is important to note that typical fenestrated vessels such as the most sinusoidal endothelial cells in the liver and the vasculature in kidney glomeruli are practically devoid of this molecule (Figure 5). In liver, only terminal portal venules, capillaries in portal tracts, and occasional terminal hepatic venules are PV-1/FELS positive. Strikingly, most sinusoidal endothelial cells remain completely negative. In kidney, the tubular veins brightly express PV-1/FELS but the fenestrated glomerular endothelium is completely devoid of this molecule (Figure 5 and data not shown).
174/2 and PAL-E recognize different epitopes. Competitive immunofluorescence stainings of tonsil. The incubations with different antibodies are indicated in the panels. Panels C and F are negative controls showing that the secondary antibodies used are isotype specific.
174/2 and PAL-E recognize different epitopes. Competitive immunofluorescence stainings of tonsil. The incubations with different antibodies are indicated in the panels. Panels C and F are negative controls showing that the secondary antibodies used are isotype specific.
PV-1/FELS antigen is not expressed on typical fenestrated endothelia in kidney glomeruli or in liver sinusoids. Liver was stained with 174/2 (A-B) or neg co, 3G6, (C) followed by FITC–anti–mouse IgG1 second-stage antibody. Kidney (D-F) was double stained with 174/2 followed by PE–anti–mouse IgG1 and FITC–anti-FVIII. The arrows point to portal tracts in panel A (higher magnification in B) and the arrow points to a glomerulus (GL) in panel D.
PV-1/FELS antigen is not expressed on typical fenestrated endothelia in kidney glomeruli or in liver sinusoids. Liver was stained with 174/2 (A-B) or neg co, 3G6, (C) followed by FITC–anti–mouse IgG1 second-stage antibody. Kidney (D-F) was double stained with 174/2 followed by PE–anti–mouse IgG1 and FITC–anti-FVIII. The arrows point to portal tracts in panel A (higher magnification in B) and the arrow points to a glomerulus (GL) in panel D.
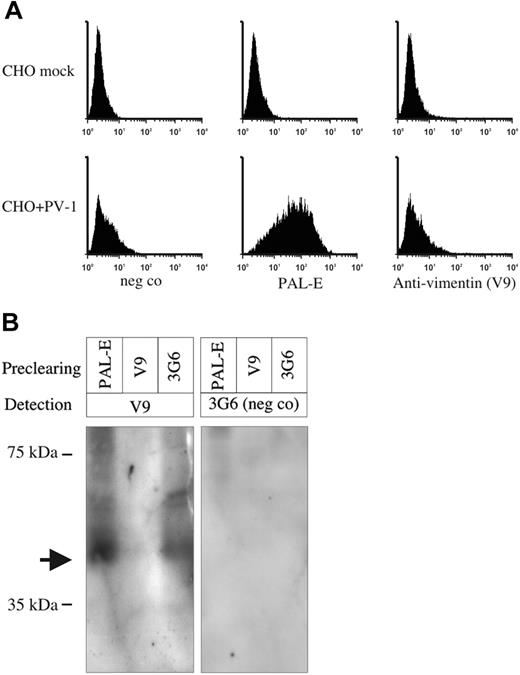
During the preparation of this work, the PAL-E antigen was reported to be a secreted form of vimentin on endothelium.4 Tissue expression of PV-1 (PAL-E staining) and vimentin argues against the two being the same molecule. To rule out the identification of PAL-E as vimentin, we stained PV-1–transfected CHO cells and mock controls with PAL-E and antivimentin antibodies. Antivimentin antibody nicely stained intracytoplasmic filaments of both PV-1– and mock-transfected controls (data not shown). In contrast, when not permeabilized, no vimentin expression was seen on the surface of either of these cells (Figure 6A). PAL-E antibody stained nonpermeabilized PV-1–transfected cells, while the mock-transfected cells remained completely negative (Figure 6A). To demonstrate that PAL-E does not recognize vimentin, we depleted PV-1–positive human microvascular endothelial cell (HMEC)–cell lysate with beads coupled to antivimentin antibody (V9), 174/2, PAL-E, or negative control antibody. Only V9 was able to remove the reactivity of V9 when the depleted lysates were tested in immunoblotting (Figure 6B). These results unambiguously demonstrate that PAL-E antigen is the same as PV-1 and not vimentin.
Discussion
In this work, we have resolved the identity of the molecule recognized by PAL-E antibody. From a historical point of view, it is interesting that PAL-E has been used so extensively and for such a long time as a marker molecule for vascular endothelium without knowing its molecular identity. It is important to note that the PAL-E antibody works only in limited applications. In contrast, the properties of the 174/2 antibody allow it to be used not only as a marker for endothelial cells but also in many other applications. Moreover, purification of the 174/2 antigen should facilitate the production of mAbs against this antigen, which also work in formalin-fixed, paraffin-embedded sections.
In a recent report, PAL-E antigen was proposed to be a secreted form of vimentin.4 There may be several reasons for the remarkable discrepancy between our results and those of Xu et al.4 First, the PAL-E antibody clone is most likely the same in both laboratories, but the antibody batch of Xu et al4 was produced as an ascites fluid. A disadvantage of this production method is that it may bring contaminating antibody specificities (eg, autoantibodies) to the actual preparation. Strangely enough, Xu et al4 report the antibody class of PAL-E to be IgG2b, although they have used the original clone that is IgG2a.12 This may speak for the impurities in the antibody preparation. Another possibility is that such an abundant protein as vimentin has come as an impurity in immunoprecipitations without direct binding to the specific antibody. Practically identical molecular weights of vimentin and PAL-E increase the difficulty of discriminating the molecules in standard electrophoresis. Our studies (Figure 6) with PV-1–transfected CHO cells and their mock controls followed by surface staining with PAL-E and the same antivimentin antibody that Xu et al4 used argue against the identity of PAL-E with any form of vimentin. Although a theoretic possibility remains even after protein sequencing, transfections to different host cell lines, double color stainings, and cross-immunoprecipitations that PAL-E and PV-1 are different molecules that are present as tight heterodimers in these assays, this is highly unlikely.
The vascular staining pattern of PAL-E is quite unique among the endothelial-cell antigens, because most of the others are shared with other cell types. For example, besides endothelium, intercellular adhesion molecule-1 (ICAM-1) recognizes lymphocytes, fibroblasts, and epithelial cells; P-selectin is also present on platelets; E-selectin is expressed on astrocytes; and vascular cell adhesion molecule (VCAM-1) is on smooth muscle cells and follicular dendritic cells.13,14 Therefore, PAL-E is a useful marker to discriminate between vascular and lymphatic endothelial cells. However, PAL-E stains only certain subtypes of blood vessels. Whether these PAL-E–positive vessels have some unique properties among the vasculature remains to be elucidated. Due to the properties of 174/2, it may be a valuable tool to analyze those vessels and further help in elucidating the function of PV-1/FELS protein, especially in pathologic processes where its expression is altered.
PAL-E antigen is not vimentin. (A) PV-1–transfected CHO cells and their mock-transfected controls were surface-stained with the antibodies indicated in the figure and analyzed by flow cytometry. The x-axis represents the fluorescence intensity, and y-axis represents the relative number of cells. (B) HMEC lysate was precleared with the indicated antibodies; thereafter, the samples were run on SDS-PAGE and blotted to nitrocellulose membranes. Detection was performed with antivimentin antibody (V9) or with a negative control antibody (3G6). Molecular weight markers are indicated on the left. The arrow points to the vimentin band.
PAL-E antigen is not vimentin. (A) PV-1–transfected CHO cells and their mock-transfected controls were surface-stained with the antibodies indicated in the figure and analyzed by flow cytometry. The x-axis represents the fluorescence intensity, and y-axis represents the relative number of cells. (B) HMEC lysate was precleared with the indicated antibodies; thereafter, the samples were run on SDS-PAGE and blotted to nitrocellulose membranes. Detection was performed with antivimentin antibody (V9) or with a negative control antibody (3G6). Molecular weight markers are indicated on the left. The arrow points to the vimentin band.
The name FELS is somewhat misleading for this protein because of the lack of PAL-E and 174/2 staining in liver sinusoidal endothelial cells and vessels in glomeruli, which are typical examples of fenestrated vessels. Therefore, we suggest that PVLAP (based on the name of the gene encoding this protein) should be used in the future as the name of this molecule.
Prepublished online as Blood First Edition Paper, August 11, 2005; DOI 10.1182/blood-2005-01-0254.
Supported by the Finnish Academy, the Finnish Cancer Organization, and the Sigrid Juselius Foundation.
H.N., K.E., and T.H. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Craig Stolen for advice and revising the language of the paper, Mikko Ares for HMEC-1 cells, Mari Parsama and Laila Reunanen for technical assistance, and Anne Sovikoski-Georgieva for secretarial help.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal