Abstract
Notch receptors are involved in lineage decisions in multiple developmental scenarios, including hematopoiesis. Here, we treated hybrid human-mouse fetal thymus organ culture with the γ-secretase inhibitor 7 (N-[N-(3,5-difluorophenyl)-l-alanyl]-S-phenyl-glycine t-butyl ester) (DAPT) to establish the role of Notch signaling in human hematopoietic lineage decisions. The effect of inhibition of Notch signaling was studied starting from cord blood CD34+ or thymic CD34+CD1-, CD34+CD1+, or CD4ISP progenitors. Treatment of cord blood CD34+ cells with low DAPT concentrations results in aberrant CD4ISP and CD4/CD8 double-positive (DP) thymocytes, which are negative for intracellular T-cell receptor β (TCRβ). On culture with intermediate and high DAPT concentrations, thymic CD34+CD1- cells still generate aberrant intracellular TCRβ- DP cells that have undergone DJ but not VDJ recombination. Inhibition of Notch signaling shifts differentiation into non-T cells in a thymic microenvironment, depending on the starting progenitor cells: thymic CD34+CD1+ cells do not generate non-T cells, thymic CD34+CD1- cells generate NK cells and monocytic/dendritic cells, and cord blood CD34+Lin- cells generate B, NK, and monocytic/dendritic cells in the presence of DAPT. Our data indicate that Notch signaling is crucial to direct human progenitor cells into the T-cell lineage, whereas it has a negative impact on B, NK, and monocytic/dendritic cell generation in a dose-dependent fashion.
Introduction
T cells develop from pluripotent hematopoietic stem cells (HSCs) through a series of differentiation steps. In humans, differentiating thymocytes can be divided into 4 main subsets based on their expression of CD4 and CD8 coreceptors. The most immature thymocytes are the CD34+ CD4-CD8- double-negative (DN) cells. As a first step toward the expression of a functional T-cell receptor (TCR), CD4+CD3- immature single-positive (CD4ISP) cells start to rearrange the TCRB gene. Subsequently, the TCRβ chain becomes assembled into the pre-TCR complex with the invariant pre-Tα chain. Pre-TCR signaling confers survival and allows development to proceed through a CD4+CD8+TCRlow double-positive (DP) subset of thymocytes. Finally, positive and negative selection results in the maturation of major histocompatibility complex (MHC)-restricted CD4 or CD8 single-positive (SP) T cells.1,2
Multiple signal transduction pathways are involved in directing cell fate decisions during T-cell development. These include regulation by Notch proteins, a family of highly conserved transmembrane receptors.3 Notch is a key player in T-cell development, and its role has been recently reviewed.4-6 There is good evidence that in mice, reduced signaling through Notch1 causes an early block in T-cell development and results in the expansion of immature B cells.7-9 However, the role of Notch1 in later stages of T-cell development is contentious. Although earlier data pointed toward a role for Notch1 in the lineage decision between αβ and γδ T cells and between CD4 and CD8 SP thymocytes,10-12 analysis of 2 different conditional Notch1 knockout mouse strains has failed to confirm these findings.13,14 This strongly argues against an important role for Notch1 in later stages of thymocyte development, though the possibility remains that other family members compensate for the lack of Notch1.
The role of Notch proteins in lymphocyte development is almost exclusively based on experiments in mice. However, it is widely accepted that differences between the thymuses of mice and humans exist,15 including the expression of cell-surface markers such as CD25 on early progenitors and the subdivision of the DN stage by CD44 and CD25.16 The role of Notch in human T-cell differentiation has only been addressed by overexpression of the active form of Notch1 in CD34+ progenitors to evaluate its influence on T-cell differentiation in hybrid human-mouse fetal thymus organ culture (FTOC).17,18 More recently, it was shown that human T-cell differentiation starting from CD34+ stem cells can be supported by OP-9 stromal cells engineered to express the Notch ligand Delta-like-1.19,20 In light of these findings, it is important to evaluate the necessity of Notch in human hematopoietic lineage decisions and thymocyte development. It has been observed that presenilin-dependent γ-secretase, which serves to cleave amyloid precursor proteins in neuronal cells, also catalyzes the release of the intracellular domain of Notch proteins.21,22 Several compounds that inhibit γ-secretase and, consequently, Notch cleavage are available and can be used to study the role of Notch signaling.23,24 In this paper, we show that the inhibition of Notch signaling in hybrid human-mouse FTOC impairs the development of human T cells and biases, in a dose-dependent way, development toward B-cell, NK-cell, and monocytic/dendritic-cell differentiation. Thus, our findings show for the first time CD34+ precursor cells as an important site of Notch action in the human thymus.
Materials and methods
Cells and sorting
Pediatric thymuses and cord blood (CB) samples were obtained and were used according to the guidelines of the Medical Ethical Commission of Ghent University Hospital (Belgium). CD34+ CB cells were purified by positive selection with CD34 magnetically activated cell sorter (MACS) beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and stained with CD34-antigen-presenting cells (APCs), CD3-fluorescein isothiocyanate (FITC), CD19-FITC, and CD56-phycoerythrin (PE) (all monoclonal antibodies [mAbs] from BD Immunocytometry Systems [BDIS], Mountain View, CA) to sort CD34+Lin- cells by flow cytometry (FACSVantage; BDIS). CD34+ thymocytes were purified by positive selection with CD34 MACS beads, stained with CD34-APC and CD1-PE, and sorted for CD1-CD34+ and CD1+CD34+ progenitors. CD4+ISP thymocytes were enriched by negative depletion of CD8+CD3+ thymocytes using DynalBeads (Dynal, Hamburg, Germany) and were labeled with CD4-PE and CD3-FITC, CD8-FITC, HLA-DR-FITC, and CD34-APC to sort CD4+ CD34-CD3-CD8-HLA-DR- cells. Purity of the cells was always at least 98%.
FTOC and flow cytometry
The mixed human-mouse FTOC was performed as described previously.25,26 FTOCs were cultured with the γ-secretase inhibitor 7 (N-[N-(3,5-difluorophenyl)-l-alanyl]-s-phenyl-glycine t-butyl ester] (DAPT; Peptides International Inc, Louisville, KY) at various concentrations, as indicated, or with the solvent dimethyl sulfoxide (DMSO). Half the medium was changed weekly. After different time points of FTOC, harvested thymocytes were incubated with anti-mouse FcRγII/III (clone 2.4.G2)27 and human immunoglobulin G (IgG) (FcBlock; Miltenyi) to avoid nonspecific staining. Cells were stained with anti-mouse CD45-CyChrome (BD PharMingen, San Diego, CA) in combination with one or more of the following anti-human mAbs: CD8β-PE, TCR-αβ-PE, CD79a-PE, CD56-APC (Coulter, Miami, FL), CD34-APC, TCR-γδ-FITC, CD3-APC or -FITC, CD4-APC or -PE, CD14-PE, CD19-FITC or PE, CD20-FITC, CD7-FITC, CD10-PE, CD56-PE, CD69-PE, HLA-DR-APC, or the appropriate control mAb (IgG1 and IgG2a-FITC, APC, or PE) (BDIS). Human viable cells, gated by exclusion of propidium iodide and mouse CD45+ cells, were examined for the expression of the antigens on a FACScalibur using CellQuest Pro software (BDIS). For intracellular staining, cells were fixed and permeabilized using Fix and Perm (Imtec, San Francisco, CA) according to the manufacturer's instructions and were stained with anti-TCRβ1-PE (Ancell, Bayport, MN), CD3-APC (BDIS), or CD79a-PE (Coulter). For DNA analysis, after staining with mAbs for surface antigens, cells underwent gentle fixation with 0.25% paraformaldehyde for 1 hour at 4°C. After washing, the cells were permeabilized with 0.05% Tween 20 for 15 minutes at 37°C, as described.28 Cells were treated with RNase (Sigma, Bornem, Belgium) and stained with 7-amino-actinomycin D (7-AAD; Becton and Dickinson) for 30 minutes at 25°C. Doublets were excluded by discriminating red fluorescence channel area × width pulses, and DNA content was measured. Apoptotic cells were detected using the annexin V-PE labeling kit from BD PharMingen.
Gene expression analysis by real-time RT-PCR
Total RNA from thymocytes was isolated as described.29 cDNA synthesis was performed by oligo(dT) priming, and real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed as described.29 Hypoxanthine guanine phosphoribosyl transferase (HPRT) mRNA was used for normalization. Human-specific primers were selected using Primer Express software (Applied Biosystems, Foster City, CA) and are shown in Supplemental Table S1, which is available at the Blood website (see the Supplemental Materials link at the top of the online article). PCR was performed with an annealing temperature of 60°C. Comparative quantification of the target gene expression in the samples was performed based on cycle threshold (Ct) normalized to HPRT using the ΔΔCt method.30
PCR analysis of TCRB gene locus recombination
DNA was extracted with the QIAmp DNA minikit (Qiagen, Hilden, Germany), and 50 ng was used for amplification by PCR. PCR conditions were performed as described31 under the following conditions: 5 minutes at 94°C, 37 cycles of 60 seconds at 94°C, 60 seconds at 63°C (DJβ amplification) or 58°C (VDJβ amplification), followed by 7 minutes at 72°C. Primers for DJβ corresponded to bases 44 to 63 (TBF1) and 3025 to 3006 (TBR1), as previously reported.32,33 For VDJβ amplification, a mixture of 5′ primers specific for Vβ2-5-8-13 families was used with TBR1. Primers are listed in Table S1 and are shown in Figure 4.
Southern blot analysis
PCR products were run on a 2% agarose gel in 1× Tris-acetate EDTA (ethylenediaminetetraacetic acid) buffer. Specificity of the amplified fragments was validated by their predicted size and Southern blot analysis. Gels were blotted in alkaline buffer (0.4 N NaOH) onto Hybond N+ membranes (Amersham, Little Chalfont, United Kingdom). Membranes were hybridized with a biotinylated TBR3 probe for 16 hours at 55°C, washed, revealed with streptavidin-horseradish peroxidase and the enhanced chemiluminescence (ECL) advanced detection system (Amersham), and visualized using x-ray film (Eastman Kodak, Rochester, NY).
Results
Notch signaling can be blocked by the γ-secretase inhibitor DAPT in hybrid human-mouse FTOC
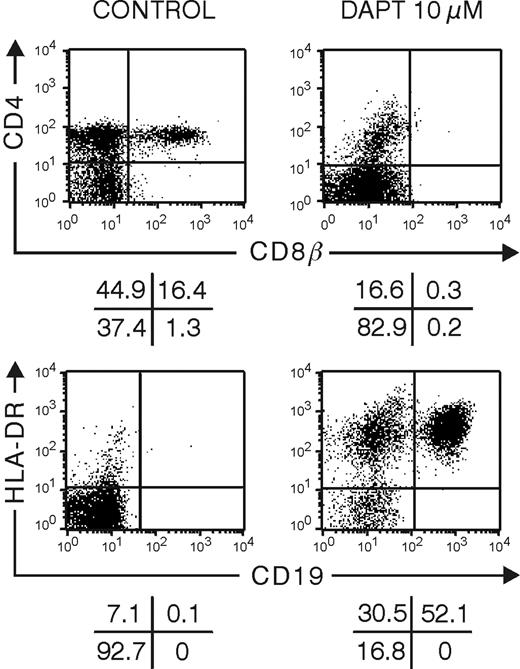
The efficiency of the γ-secretase inhibitor DAPT to block Notch signaling in hybrid human-mouse FTOC was assessed by its effect on thymocyte development and expression of the Notch target gene HES1. Individual thymic lobes obtained from embryonic day 14 to 15 mouse SCID-NOD fetuses were seeded with purified human CB CD34+ progenitor cells, cultured for 28 days in the absence or presence of 10 μM DAPT, and analyzed by flow cytometry (Figure 1). Control FTOC contained a significant number of DP cells, which were absent in DAPT-treated cultures. In contrast, DAPT-treated FTOCs were, to a large degree, composed of CD19+ HLA-DR+ B cells, which were virtually absent in control cultures (Figure 1). This is in agreement with previous findings in mice showing that inactivation of Notch allows B-cell development intrathymically.7,8 To assess the effect of DAPT on HES1 expression, we cultured FTOCs that were seeded with CD34+ thymocytes for 10 days with medium alone before a 5-day culture period in the absence or presence of DAPT. Given that in this condition DAPT treatment has only a moderate effect on cellular composition (data not shown), the direct unbiased effect of DAPT on Hes-1 expression could be assessed. HES1 mRNA levels were reduced by 80% in DAPT-treated cultures (ΔΔCt = 2.31 ± 0.06, mean ± SEM, for 3 independent experiments).11,34 FTOCs seeded with CD34+CD1- thymocytes and cultured for 4 days before a short-term culture of 18 hours with vehicle alone or with 2, 5, or 10 μM DAPT showed a DAPT dose-dependent reduction in HES1 by 41%, 53%, and 54%, respectively, which was statistically significant (paired t test) between control and DAPT-treated cultures (P < .01) and between 2 μM and 5 μM DAPT-treated cultures (P < .02). Our results clearly demonstrate that the γ-secretase inhibitor DAPT is capable of blocking Notch signaling in hybrid human-mouse FTOC.
DAPT inhibits Notch signaling in hybrid human-mouse fetal thymus organ culture. Representative flow cytometric analyses of human cells from FTOC seeded with human CD34+ CB progenitor cells and cultured for 28 days in the absence or presence of 10 μM DAPT. Quadrants were set according to isotype controls.
DAPT inhibits Notch signaling in hybrid human-mouse fetal thymus organ culture. Representative flow cytometric analyses of human cells from FTOC seeded with human CD34+ CB progenitor cells and cultured for 28 days in the absence or presence of 10 μM DAPT. Quadrants were set according to isotype controls.
Inhibition of Notch signaling in FTOC of CB CD34+ progenitor cells arrests T-cell development and shifts differentiation to B, NK, and monocytic-dendritic cells in a dose-dependent way
Because DAPT did not interfere with the entry of the precursor cells into the thymic lobes (data not shown), we performed the experiments by adding DAPT from the start, including the hanging drop. Half the medium was changed weekly to maintain the concentration of active product. Inhibition of Notch with 2 and 10 μM DAPT did not result in a significant change in the absolute human cell numbers generated in FTOC after 28 days. Interestingly, there was a significant decrease in the total cell number when the FTOC was treated with an intermediate dose of 5 μM DAPT (Table 1). This observation must be interpreted in view of the change in the cellular composition and the cell-specific generation potential.
Influence of γ-secretase inhibition by DAPT on human cellularity and cell number of human subpopulations after 28 days of FTOC seeded with human CD34+Lin- cord blood progenitor cells
. | . | DAPT treatment . | . | . | ||
|---|---|---|---|---|---|---|
| Cell type . | Control, no. cells . | 2 μM, no. cells . | 5 μM, no. cells . | 10 μM, no. cells . | ||
| Human cells | 55 645 ± 8 676 | 41 591 ± 6 869 | 24 541 ± 3 191* | 32 089 ± 1 755 | ||
| CD34+ | 5 618 ± 1 469 | 1 792 ± 736* | 1 411 ± 110* | 1 930 ± 188 | ||
| CD7+ | 50 646 ± 8 137 | 27 448 ± 9 725* | 8 531 ± 2 563* | 4 533 ± 1 060* | ||
| CD4ISP | 24 828 ± 3 974 | 18 487 ± 4 598 | 915 ± 596* | 0 ± 0* | ||
| DP | 8 562 ± 3 238 | 2 549 ± 951 | 345 ± 173* | 0 ± 0* | ||
| IC CD3+ | 53 119 ± 8 319 | 35 414 ± 6 671 | 5 333 ± 1 856* | 518 ± 326* | ||
| IC TCR-β | 14 103 ± 3 490 | 344 ± 210* | 25 ± 25* | 0 ± 0* | ||
| CD3+ | 10 952 ± 5 146 | 1 474 ± 737* | 21 ± 21* | 0 ± 0* | ||
| TCR-αβ | 8 449 ± 2 740 | 291 ± 212* | 0 ± 0* | 0 ± 0* | ||
| TCR-γδ | 2 479 ± 313 | 294 ± 114* | 0 ± 0* | 0 ± 0* | ||
| CD4+HLA-DR+ | 1 614 ± 266 | 1 391 ± 496 | 2 640 ± 502 | 5 266 ± 790* | ||
| CD14+ | 231 ± 75 | 241 ± 60 | 533 ± 107* | 1 352 ± 356* | ||
| CD56+ | 1 247 ± 656 | 2 052 ± 379 | 4 324 ± 1 134* | 3 079 ± 861 | ||
| CD19+ | 38 ± 45 | 454 ± 171* | 6 516 ± 2 219* | 17 357 ± 3 052* | ||
. | . | DAPT treatment . | . | . | ||
|---|---|---|---|---|---|---|
| Cell type . | Control, no. cells . | 2 μM, no. cells . | 5 μM, no. cells . | 10 μM, no. cells . | ||
| Human cells | 55 645 ± 8 676 | 41 591 ± 6 869 | 24 541 ± 3 191* | 32 089 ± 1 755 | ||
| CD34+ | 5 618 ± 1 469 | 1 792 ± 736* | 1 411 ± 110* | 1 930 ± 188 | ||
| CD7+ | 50 646 ± 8 137 | 27 448 ± 9 725* | 8 531 ± 2 563* | 4 533 ± 1 060* | ||
| CD4ISP | 24 828 ± 3 974 | 18 487 ± 4 598 | 915 ± 596* | 0 ± 0* | ||
| DP | 8 562 ± 3 238 | 2 549 ± 951 | 345 ± 173* | 0 ± 0* | ||
| IC CD3+ | 53 119 ± 8 319 | 35 414 ± 6 671 | 5 333 ± 1 856* | 518 ± 326* | ||
| IC TCR-β | 14 103 ± 3 490 | 344 ± 210* | 25 ± 25* | 0 ± 0* | ||
| CD3+ | 10 952 ± 5 146 | 1 474 ± 737* | 21 ± 21* | 0 ± 0* | ||
| TCR-αβ | 8 449 ± 2 740 | 291 ± 212* | 0 ± 0* | 0 ± 0* | ||
| TCR-γδ | 2 479 ± 313 | 294 ± 114* | 0 ± 0* | 0 ± 0* | ||
| CD4+HLA-DR+ | 1 614 ± 266 | 1 391 ± 496 | 2 640 ± 502 | 5 266 ± 790* | ||
| CD14+ | 231 ± 75 | 241 ± 60 | 533 ± 107* | 1 352 ± 356* | ||
| CD56+ | 1 247 ± 656 | 2 052 ± 379 | 4 324 ± 1 134* | 3 079 ± 861 | ||
| CD19+ | 38 ± 45 | 454 ± 171* | 6 516 ± 2 219* | 17 357 ± 3 052* | ||
Results represent mean ± SEM of 4 independent experiments.
Significant difference (P < .05; paired Student t test) compared with the vehicle-treated control cultures
The frequency and number of CD34+ cells, which represent the most immature cells, were significantly reduced by more than 50% in cultures with 2 and 5 μM of DAPT (Table 1 and Table S2), compatible with the view that Notch supports the maintenance of CD34+ progenitor cells.35 At a dose of 10 μM, the frequency and total number of CD34+ cells tended to be lower, but were no longer significantly decreased (Table 1 and Table S2). It is possible that the efficient generation of B cells in this condition, which we will present further, is accompanied by an increase in CD34+ B cell-committed progenitors.
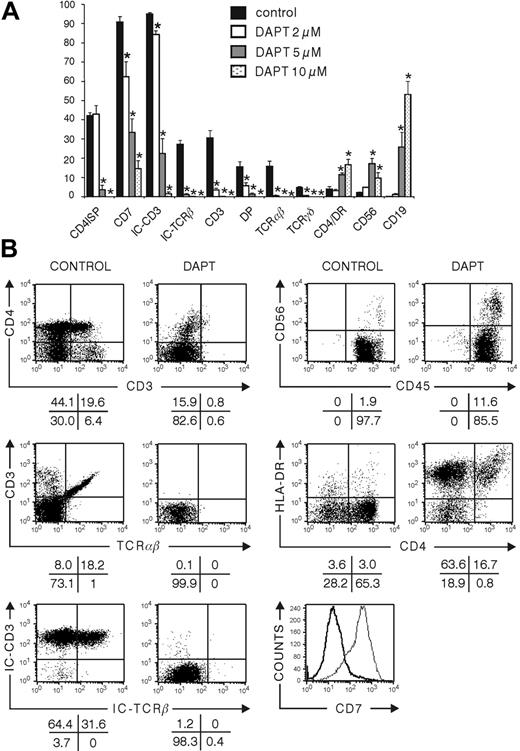
In mice, the inhibition of Notch signaling results in the accumulation of B cells.7 FTOC seeded with CB CD34+Lin- precursor cells showed a significant dose-dependent increase in frequency and in absolute numbers of B cells after the inhibition of Notch signaling by DAPT (Figure 2A), varying from 12- to 460-fold, depending on the dose of DAPT (Table 1). After 28 days of culture with the highest concentration of DAPT, B cells represented the most predominant population, encompassing more than 50% of all human cells (Figure 1), which were positive for CD10 and CD20 and partly positive for intracellular CD79a (Figure S1A).
In rat FTOC, inhibition of Notch by DAPT results in a dramatic increase in the number of NK cells.34 Here, DAPT-treated FTOC showed a significant increase in the frequency and number of cells positively staining for CD56, which is expressed on all human NK cells (Figure 2A-B; Table 1), from less than 3% in controls to approximately 10% with the highest concentration of DAPT, corresponding to a 2.5-fold increase in the absolute number of CD56+ cells (Figure 2A; Table 1). At a lower concentration of the inhibitor (5 μM), the frequency of the number of CD56+ cells increased to more than 17%, corresponding to more than a 3.5-fold increase in absolute number of CD56+ cells (Figure 2A; Table 1). Most of the CD56+ cells were negative for CD3, CD4 (Figure 2A), and CD8β surface expression (data not shown) and positive for CD7 (Figure S1B). This corresponds to the phenotype of mature peripheral NK cells and argues that the CD56+ cells in DAPT-treated cultures represent true NK cells.36 On activation, NK cells are known to up-regulate triggering receptors such as CD69 on their surfaces, endowing them with new recognition capabilities.37 Most CD56+ cells in DAPT-treated fetal thymuses were negative for CD69 surface expression, suggestive of a nonactivated phenotype (Figure S1B). Collectively, this suggests that in the absence of Notch signaling, NK-cell development from CD34+ progenitors is favored. At the highest concentration (10 μM) of DAPT, CD34+ progenitor cells are preferentially biased toward B cells, whereas in the presence of intermediate concentrations, the CD34+ cells are less efficiently skewed toward B-cell differentiation but are able to generate NK cells. At low concentrations of DAPT, the generation of NK cells is still favored compared with untreated cultures, but not to the same extent as with intermediate DAPT concentrations.
Influence of DAPT inhibition of Notch signaling on differentiation of CD34+ CB cells in hybrid human-mouse FTOC. Murine fetal thymic lobes were seeded with human CD34+ CB cells and cultured in FTOC for 28 days in the absence of presence of 2, 5, or 10 μM DAPT. (A) Frequencies of subpopulations of human hematopoietic cells at the end of the culture period. Bars represent mean ± SEM of 4 independent experiments. Asterisks indicate statistically significant differences (P < .05) compared with vehicle-treated control. (B) Representative dot plots and histogram of flow cytometric analysis of control cultures and cultures treated with 10 μM DAPT. Bold line and thin line in the histogram represent results from the DAPT-treated and control culture, respectively.
Influence of DAPT inhibition of Notch signaling on differentiation of CD34+ CB cells in hybrid human-mouse FTOC. Murine fetal thymic lobes were seeded with human CD34+ CB cells and cultured in FTOC for 28 days in the absence of presence of 2, 5, or 10 μM DAPT. (A) Frequencies of subpopulations of human hematopoietic cells at the end of the culture period. Bars represent mean ± SEM of 4 independent experiments. Asterisks indicate statistically significant differences (P < .05) compared with vehicle-treated control. (B) Representative dot plots and histogram of flow cytometric analysis of control cultures and cultures treated with 10 μM DAPT. Bold line and thin line in the histogram represent results from the DAPT-treated and control culture, respectively.
FTOC seeded with human CB CD34+ progenitors and cultured in high (10 μM) DAPT concentrations resulted in the emergence of a population with characteristics of monocytic and dendritic cells. These cells coexpressed CD4 and HLA-DR and had relatively high side scatter, and some of them were CD14+ (Figure 2B and data not shown). The absolute numbers of CD4+HLA-DR+ cells and CD14+ cells were significantly increased in FTOC treated with high and intermediate concentrations of DAPT and resulted in a more than 3-fold increase (Table 1).
In control FTOCs, CB CD34+ progenitors undergo T-cell maturation in the mouse thymic microenvironment. This is shown by the progress of CD34+ cells into CD4ISP cells, which are immature thymocytes that start to rearrange TCRB. After 28 days of culture, more than 40% of the human cells achieved this maturation stage (Figure 2A-B). Approximately 15% of the cells already matured to a further stage, with coexpression of CD4+CD8 and surface expression of CD3 (Figure 2A-B). The frequency of cells that stain for intracellular TCRβ exceeds that of DP thymocytes, as part of CD4ISP, and CD4+CD8αα+ cells express intracellular TCRβ.38 FTOC with human CB CD34+ progenitors displayed a complete block of T-cell differentiation at high DAPT concentrations. Although a low number of CD4+CD3- cells appeared (Figure 2B), those cells were not CD4ISP immature T-cell precursors; rather, they belonged to the monocytic/dendritic cell lineage according to the coexpression of HLA-DR, the absence of intracellular CD3 (Figure 2A-B), and the different scatter properties (data not shown). At intermediate DAPT concentrations, CD34+ CB progenitor cells were able to progress toward T cells, but the number of CD4ISP and DP cells was extremely low. At low DAPT concentrations, the number of CD4ISP and DP cells tended to be lower than for the control, but there was no significant difference. However, some striking differences were observed in the 2 μM DAPT-treated cultures. One was the nearly complete absence of intracellular TCR-β, and the other was a significant reduction in CD7 expression (Table 1).
Inhibition of Notch signaling in CD34+CD1- thymic progenitor cells does not lead to the generation of B lymphocytes, allows NK- and monocytic/dendritic-cell maturation, and results in differentiation of abnormal T lymphocytes in FTOC
To have a better view of the effect of Notch inhibition on T-cell maturation, we explored the influence of DAPT on FTOC seeded with CD34+ progenitors purified from human thymocytes. Those progenitor cells have already entered the thymus and received Notch signaling39 and are more efficient generating large numbers of human T cells with faster kinetics in FTOC.
In contrast to FTOCs that were seeded with CB CD34+ cells, those seeded with CD34+CD1- thymic precursors were unable to generate B cells, even at the highest DAPT concentration (data not shown). This is compatible with the view that CD34+ progenitor cells in the thymus have received Notch triggering and possibly other signals that have already irreversibly induced the CD34+ precursor cell to a stage wherein the capacity to develop into B cells is lost, but they retained their capacity to develop into NK cells. The absolute number of NK cells was the highest with intermediate concentrations of the inhibitor (Tables 2, 3). Surprisingly, and in contrast to the CB CD34+ cultures, there was a significant dose-dependent increase in the frequency and number of DP cells generated in DAPT-treated FTOC. However, those DP cells were abnormal because they lacked surface CD3 and intracellular TCR-β expression (Figure 3A; Tables 2, 3). Furthermore, the inhibition of Notch triggering in CD34+ thymic progenitors leads to a reduction of CD7 in a concentration-dependent manner (Figure 3B).
Influence of γ-secretase inhibition by DAPT on human cellularity of cell subsets in FTOC seeded with human CD34+CD1- thymic progenitor cells after 13 and 19 days of culture
Days after treatment, cell type . | Control, no. cells . | DAPT treatment . | . | |
|---|---|---|---|---|
| . | . | 5 μM, no. cells . | 10 μM, no. cells . | |
| After 13 days | ||||
| Human cells | 61 873 ± 10 604* | 85 730 ± 25 356 | 92 214 ± 4 988 | |
| CD4ISP | 36 481 ± 5 404 | 38 364 ± 11 521 | 43 343 ± 2 869 | |
| DP | 12 618 ± 6 568 | 52 810 ± 16 178† | 62 758 ± 3 316† | |
| IC TCR-β | 21 176 ± 8 391 | 891 ± 340† | 623 ± 343† | |
| CD3+ | 14 467 ± 5 176 | 723 ± 426† | 596 ± 292† | |
| TCR-αβ | 9 571 ± 3 505 | 413 ± 267† | 456 ± 271† | |
| TCR-γδ | 3 035 ± 893 | 140 ± 166† | 71 ± 62† | |
| After 19 days | ||||
| Human cells | 208 397 ± 48 574 | 44 868 ± 7 632† | 18 731 ± 7 118† | |
| CD56+ | 377 ± 72 | 1 408 ± 343† | 1 285 ± 770 | |
| CD4ISP | 62 544 ± 15 294 | 7 012 ± 2 060† | 1 933 ± 365† | |
| DP | 130 330 ± 40 609 | 30 574 ± 6 641† | 16 843 ± 3 806† | |
| IC TCR-β | 125 048 ± 30 095 | 2 291 ± 730† | 689 ± 609† | |
| CD3+ | 109 360 ± 528 583 | 2 301 ± 448† | 1 128 ± 125† | |
| TCR-αβ | 89 242 ± 20 756 | 1 342 ± 263† | 630 ± 92† | |
| TCR-γδ | 6 096 ± 1 365 | 214 ± 66† | 192 ± 88† | |
Days after treatment, cell type . | Control, no. cells . | DAPT treatment . | . | |
|---|---|---|---|---|
| . | . | 5 μM, no. cells . | 10 μM, no. cells . | |
| After 13 days | ||||
| Human cells | 61 873 ± 10 604* | 85 730 ± 25 356 | 92 214 ± 4 988 | |
| CD4ISP | 36 481 ± 5 404 | 38 364 ± 11 521 | 43 343 ± 2 869 | |
| DP | 12 618 ± 6 568 | 52 810 ± 16 178† | 62 758 ± 3 316† | |
| IC TCR-β | 21 176 ± 8 391 | 891 ± 340† | 623 ± 343† | |
| CD3+ | 14 467 ± 5 176 | 723 ± 426† | 596 ± 292† | |
| TCR-αβ | 9 571 ± 3 505 | 413 ± 267† | 456 ± 271† | |
| TCR-γδ | 3 035 ± 893 | 140 ± 166† | 71 ± 62† | |
| After 19 days | ||||
| Human cells | 208 397 ± 48 574 | 44 868 ± 7 632† | 18 731 ± 7 118† | |
| CD56+ | 377 ± 72 | 1 408 ± 343† | 1 285 ± 770 | |
| CD4ISP | 62 544 ± 15 294 | 7 012 ± 2 060† | 1 933 ± 365† | |
| DP | 130 330 ± 40 609 | 30 574 ± 6 641† | 16 843 ± 3 806† | |
| IC TCR-β | 125 048 ± 30 095 | 2 291 ± 730† | 689 ± 609† | |
| CD3+ | 109 360 ± 528 583 | 2 301 ± 448† | 1 128 ± 125† | |
| TCR-αβ | 89 242 ± 20 756 | 1 342 ± 263† | 630 ± 92† | |
| TCR-γδ | 6 096 ± 1 365 | 214 ± 66† | 192 ± 88† | |
Results represent mean ± SEM of 3 to 5 independent experiments.
Absolute cell number
Significant difference (P < .05; paired Student t test) compared with vehicle-treated control cultures
Influence of γ-secretase inhibition by DAPT on frequency of cell subsets in FTOC seeded with human CD34+CD1- thymic progenitor cells after 13 and 19 days of culture
. | . | DAPT treatment . | . | |
|---|---|---|---|---|
| Cell subset . | Control . | 5 μM . | 10 μM . | |
| After 13 days | ||||
| CD4ISP | 57.3 ± 1.7 | 43.0 ± 2.6 | 35.3 ± 0.8 | |
| DP | 14.7 ± 6.0 | 52.6 ± 5.4* | 56.7 ± 0.6* | |
| IC TCR-β | 26.3 ± 7.1 | 1.3 ± 0.1* | 0.9 ± 0.2* | |
| CD3+ | 17.9 ± 4.6 | 1.0 ± 0.2* | 0.8 ± 0.2* | |
| TCR-αβ | 1.3 ± 0.3 | 0.5 ± 0.1* | 0.3 ± 0.1* | |
| TCR-γδ | 4.0 ± 0.5 | 0.2 ± 0.1* | 0.1 ± 0.0* | |
| After 19 days | ||||
| CD56+ | 0.2 ± 0.1 | 2.7 ± 0.3* | 8.3 ± 2.1* | |
| CD4ISP | 34.4 ± 6.3 | 16.6 ± 5.2* | 8.7 ± 2.0* | |
| DP | 56.3 ± 9.3 | 68.9 ± 8.8* | 71.1 ± 3.6* | |
| IC TCR-β | 63.4 ± 7.1 | 5.3 ± 1.2* | 2.1 ± 1.7* | |
| CD3+ | 50.6 ± 3.5 | 5.1 ± 0.5* | 5.1 ± 0.8* | |
| TCR-αβ | 36.8 ± 3.8 | 2.3 ± 0.6* | 2.3 ± 0.5* | |
| TCR-γδ | 3.3 ± 0.9 | 0.4 ± 0.1* | 0.9 ± 0.5* | |
. | . | DAPT treatment . | . | |
|---|---|---|---|---|
| Cell subset . | Control . | 5 μM . | 10 μM . | |
| After 13 days | ||||
| CD4ISP | 57.3 ± 1.7 | 43.0 ± 2.6 | 35.3 ± 0.8 | |
| DP | 14.7 ± 6.0 | 52.6 ± 5.4* | 56.7 ± 0.6* | |
| IC TCR-β | 26.3 ± 7.1 | 1.3 ± 0.1* | 0.9 ± 0.2* | |
| CD3+ | 17.9 ± 4.6 | 1.0 ± 0.2* | 0.8 ± 0.2* | |
| TCR-αβ | 1.3 ± 0.3 | 0.5 ± 0.1* | 0.3 ± 0.1* | |
| TCR-γδ | 4.0 ± 0.5 | 0.2 ± 0.1* | 0.1 ± 0.0* | |
| After 19 days | ||||
| CD56+ | 0.2 ± 0.1 | 2.7 ± 0.3* | 8.3 ± 2.1* | |
| CD4ISP | 34.4 ± 6.3 | 16.6 ± 5.2* | 8.7 ± 2.0* | |
| DP | 56.3 ± 9.3 | 68.9 ± 8.8* | 71.1 ± 3.6* | |
| IC TCR-β | 63.4 ± 7.1 | 5.3 ± 1.2* | 2.1 ± 1.7* | |
| CD3+ | 50.6 ± 3.5 | 5.1 ± 0.5* | 5.1 ± 0.8* | |
| TCR-αβ | 36.8 ± 3.8 | 2.3 ± 0.6* | 2.3 ± 0.5* | |
| TCR-γδ | 3.3 ± 0.9 | 0.4 ± 0.1* | 0.9 ± 0.5* | |
Results represent mean percentage ± SEM of 3 to 5 independent experiments.
Significant difference (P < .05; paired Student t test) compared with vehicle-treated control cultures
FTOC with increasing DAPT concentrations exhibited a progressive decrease in the number of mature CD3+ DP cells, together with an elevation in the number of CD4ISP cells (Tables 2, 3). Importantly, CD7 was markedly down-regulated in those cells (Figure 3B). CD7 is found primarily on T, NK, and pre-B cells. The fact that CD7 expression was unchanged on NK cells argues against unspecific loss of expression or detectability.
Inhibition of Notch signaling in differentiating CD34+CD1- thymic progenitor cells in FTOC affects VDJβ, but not DJβ, rearrangement
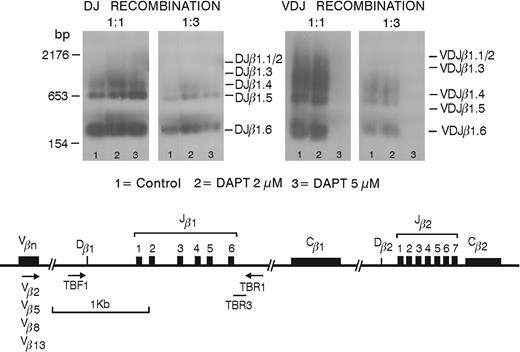
Southern blot analyses performed on human thymocytes generated in FTOC started with CD34+CD1- thymic progenitor cells. Although both cell populations had a significant number of DP thymocytes and a similar extent of DJβ rearrangement, VDJβ rearrangement of the VDJβ 2-5-8-13 genes was virtually absent at 5 μM DAPT (Figure 4).
DAPT treatment induces thymic CD34+CD1+ cells and CD4ISP cells to develop into aberrant DP cells lacking intracellular TCR-β
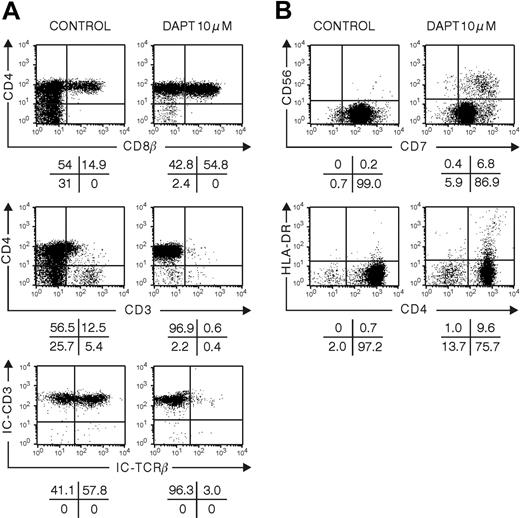
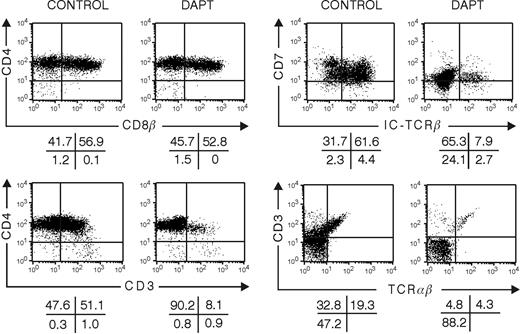
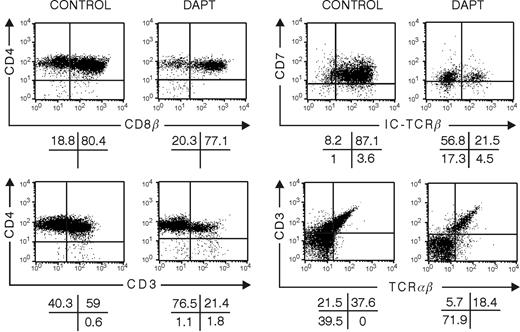
To study the impact of Notch signaling on late T-cell-lineage decisions, we grew hybrid FTOC that were colonized with thymocytes representing successive stages of differentiation. We studied the development of CD34+CD1+ and CD4ISP human thymocytes cultured for 10 or 20 days in FTOC in the absence or presence of DAPT. At a dose of 10 μM DAPT, few cells were present, precluding detailed phenotypic analysis. Therefore, FTOCs were grown at a lower dose of 5 μM. In control cultures, a frequency of DP cells was obtained that was similar to the one found in vivo and that was higher than the frequency obtained in FTOC seeded with CD34+CD1- thymic or CD34+ CB progenitor cells, which require a longer incubation period to achieve a similar degree of differentiation40 (compare control treatment in Figure 1 to that in Figures 5 and 6). This shows that FTOC supports human T-cell development irrespective of the maturation degree of the starting cells but that the kinetics depend on the differentiation stage. T-cell maturation, as characterized by TCRαβ expression, occurred normally, and less than 1% of the cells were NK cells (data not shown). Although CD34+CD1+ thymocytes were able to differentiate quickly toward T cells in control conditions, the inhibition of Notch signaling still resulted in a severe block of T-cell development with low numbers of CD3+ cells present after 10 days (Figure 5) and with a strong decrease of cells with intracellular TCR-β expression. Although the frequency of DP thymocytes was not severely changed, the absolute numbers of DP cells were dramatically decreased in the DAPT-treated cultures after 20 days (data not shown). Because no B or NK cells were generated in DAPT-treated cultures (data not shown), these results demonstrate that DAPT directly inhibits T-cell differentiation, not that inhibition of T-cell differentiation would be merely caused by a bias toward B or NK differentiation. When CD4ISP thymocytes were cultured for 10 days in FTOC in the presence of DAPT, aberrant CD4/CD8 DP cells were still obtained that were predominantly negative for surface CD3 and intracellular TCRβ (Figure 6).
Influence of DAPT treatment on differentiation of human CD34+CD1- thymic progenitor cells in human-mouse hybrid FTOC. Murine fetal thymic lobes were seeded with human CD34+CD1- thymic progenitor cells and cultured for 13 (A) or 19 (B) days in the absence or presence of 10 μM DAPT. Representative dot plots of 4 independent experiments are shown.
Influence of DAPT treatment on differentiation of human CD34+CD1- thymic progenitor cells in human-mouse hybrid FTOC. Murine fetal thymic lobes were seeded with human CD34+CD1- thymic progenitor cells and cultured for 13 (A) or 19 (B) days in the absence or presence of 10 μM DAPT. Representative dot plots of 4 independent experiments are shown.
Inhibition of Notch signaling affects VDJβ but not DJβ rearrangements. Southern blots are shown of the products of PCR performed on cell lysates of control or DAPT-treated FTOCs, which were originally seeded with CD34+CD1- human thymic progenitor cells and were cultured for 20 days. DJβ (left) and VDJβ (right) rearrangements are shown for undiluted and 1:3 dilutions of the PCR products. Lanes 1 to 3 represent samples from untreated FTOC or FTOC in the presence of 2 or 5 μM DAPT, respectively.
Inhibition of Notch signaling affects VDJβ but not DJβ rearrangements. Southern blots are shown of the products of PCR performed on cell lysates of control or DAPT-treated FTOCs, which were originally seeded with CD34+CD1- human thymic progenitor cells and were cultured for 20 days. DJβ (left) and VDJβ (right) rearrangements are shown for undiluted and 1:3 dilutions of the PCR products. Lanes 1 to 3 represent samples from untreated FTOC or FTOC in the presence of 2 or 5 μM DAPT, respectively.
Cell-cycle analysis revealed that on DAPT treatment (Table 4), more cells were cycling during the first 10 days of culture. This was also the case when gating on CD4ISP cells. At this point, the number of annexin V+ cells resembling apoptotic cells was comparable, indicating that the emergence of abnormal cells in DAPT-treated FTOC resulted from rapid outgrowth of those cells. After 20 days, however, the DAPT-treated cells stopped cycling and showed a significant increase in the number of apoptotic cells, which resulted in a dramatic decrease in cell number.
Influence of γ-secretase inhibition by DAPT on human cellularity, proliferative status, and annexin V+ cells in cell subsets in FTOC seeded with CD34+CD1- thymic progenitor cells after different time points of culture
Cell type, treatment . | Day 4 . | Day 7 . | Day 10 . | Day 20 . | Day 26 . |
|---|---|---|---|---|---|
| Cell no. | |||||
| Control | 16 391 ± 2 911 | 49 107 ± 315 | 61 303 ± 9 024 | 502 068 ± 13 762 | 828 750 |
| 2 μM DAPT | 11 389 ± 713 | 56 108 ± 4 024 | 108 889 ± 45 035 | 285 806 ± 43 782 | 148 353 |
| 5 μM DAPT | 13 016 ± 2 674 | 40 626 ± 5 763 | 123 874 ± 5 633 | 94 166 ± 75 717 | 38 500 |
| S/G2/M cells in total cell population, % | |||||
| Control | 17 ± 5.9 | 10 ± 1.4 | 12 ± 4.2 | 18 ± 2.4 | 6.2 |
| 2 μM DAPT | 22 ± 7.4 | 18 ± 4.2 | 20 ± 2.8 | 4 ± 1.2 | 1.3 |
| 5 μM DAPT | 25 ± 1.3 | 27 ± 0.8 | 15 ± 0.4 | 1 ± 0.1 | 0.5 |
| Annexin V+ cells in total cell population, % | |||||
| Control | 45 ± 12.7 | 42 ± 5.7 | 55 ± 3.5 | 38 ± 6.4 | 32 |
| 2 μM DAPT | 49 ± 14.4 | 37 ± 1.3 | 38 ± 2.0 | 32 ± 8.4 | 36 |
| 5 μM DAPT | 52 ± 16.5 | 49 ± 5.4 | 44 ± 9.1 | 64 ± 9.3 | 65 |
| S/G2/M cells in CD4+ subset, % | |||||
| Control | ND | 8 | 9 ± 2.3 | 23 ± 2.1 | 6.2 |
| 2 μM DAPT | ND | 20 | 18 ± 3.1 | 8 ± 2.6 | 1.3 |
| 5 μM DAPT | ND | 27 | 15 ± 0.6 | 2 ± 0.7 | 0.5 |
| Annexin V+ cells in CD4+ subset, % | |||||
| Control | ND | 40 ± 1.8 | 55 ± 3.6 | 51 ± 10.5 | 44 |
| 2 μM DAPT | ND | 36 ± 4.0 | 38 ± 4.6 | 41 ± 12.6 | 48 |
| 5 μM DAPT | ND | 48 ± 0.1 | 49 ± 9.2 | 66 ± 11.9 | 73 |
| S/G2/M cells in CD4+CD8+ subset, % | |||||
| Control | ND | ND | ND | 12 ± 1.7 | 3.6 |
| 2 μM DAPT | ND | ND | ND | 1 ± 0.3 | 0.5 |
| 5 μM DAPT | ND | ND | ND | 1 ± 0.3 | 0.2 |
| Annexin V+ cells in CD4+CD8+ subset, % | |||||
| Control | ND | ND | ND | 42 ± 12.6 | 41 |
| 2 μM DAPT | ND | ND | ND | 43 ± 11.0 | 51 |
| 5 μM DAPT | ND | ND | ND | 69 ± 15.8 | 74 |
Cell type, treatment . | Day 4 . | Day 7 . | Day 10 . | Day 20 . | Day 26 . |
|---|---|---|---|---|---|
| Cell no. | |||||
| Control | 16 391 ± 2 911 | 49 107 ± 315 | 61 303 ± 9 024 | 502 068 ± 13 762 | 828 750 |
| 2 μM DAPT | 11 389 ± 713 | 56 108 ± 4 024 | 108 889 ± 45 035 | 285 806 ± 43 782 | 148 353 |
| 5 μM DAPT | 13 016 ± 2 674 | 40 626 ± 5 763 | 123 874 ± 5 633 | 94 166 ± 75 717 | 38 500 |
| S/G2/M cells in total cell population, % | |||||
| Control | 17 ± 5.9 | 10 ± 1.4 | 12 ± 4.2 | 18 ± 2.4 | 6.2 |
| 2 μM DAPT | 22 ± 7.4 | 18 ± 4.2 | 20 ± 2.8 | 4 ± 1.2 | 1.3 |
| 5 μM DAPT | 25 ± 1.3 | 27 ± 0.8 | 15 ± 0.4 | 1 ± 0.1 | 0.5 |
| Annexin V+ cells in total cell population, % | |||||
| Control | 45 ± 12.7 | 42 ± 5.7 | 55 ± 3.5 | 38 ± 6.4 | 32 |
| 2 μM DAPT | 49 ± 14.4 | 37 ± 1.3 | 38 ± 2.0 | 32 ± 8.4 | 36 |
| 5 μM DAPT | 52 ± 16.5 | 49 ± 5.4 | 44 ± 9.1 | 64 ± 9.3 | 65 |
| S/G2/M cells in CD4+ subset, % | |||||
| Control | ND | 8 | 9 ± 2.3 | 23 ± 2.1 | 6.2 |
| 2 μM DAPT | ND | 20 | 18 ± 3.1 | 8 ± 2.6 | 1.3 |
| 5 μM DAPT | ND | 27 | 15 ± 0.6 | 2 ± 0.7 | 0.5 |
| Annexin V+ cells in CD4+ subset, % | |||||
| Control | ND | 40 ± 1.8 | 55 ± 3.6 | 51 ± 10.5 | 44 |
| 2 μM DAPT | ND | 36 ± 4.0 | 38 ± 4.6 | 41 ± 12.6 | 48 |
| 5 μM DAPT | ND | 48 ± 0.1 | 49 ± 9.2 | 66 ± 11.9 | 73 |
| S/G2/M cells in CD4+CD8+ subset, % | |||||
| Control | ND | ND | ND | 12 ± 1.7 | 3.6 |
| 2 μM DAPT | ND | ND | ND | 1 ± 0.3 | 0.5 |
| 5 μM DAPT | ND | ND | ND | 1 ± 0.3 | 0.2 |
| Annexin V+ cells in CD4+CD8+ subset, % | |||||
| Control | ND | ND | ND | 42 ± 12.6 | 41 |
| 2 μM DAPT | ND | ND | ND | 43 ± 11.0 | 51 |
| 5 μM DAPT | ND | ND | ND | 69 ± 15.8 | 74 |
Results represent mean ± SEM of 2 independent experiments, except for figures without SEM shown, when only 1 experiment was performed.
ND indicates not done.
Discussion
We demonstrate that Notch signals are required for human T-cell development and that this regulation bears many similarities to murine T-cell development. We used the γ-secretase inhibitor DAPT to interfere with Notch signaling and to assess its role in human T-cell differentiation. Although this approach does not target the human developing cells exclusively but may affect the murine fetal thymic stromal cells and is not entirely specific for the Notch pathway, our results are consistent with the assumption that the observed effects can be ascribed to the inhibition of Notch signaling in human cells. First, there was a decrease in the downstream target gene HES1 in the human cells. Second, our results are consistent with those of the conditional Cre-lox knockout mice in which only Notch1 in the lymphoid cells was targeted and the stromal cells were intact.7
Influence of the inhibition of Notch signaling with DAPT on development in hybrid human-mouse FTOC seeded with human CD34+CD1+ thymic progenitor cells. Murine fetal thymic lobes were seeded with human CD34+CD1+ thymic progenitor cells cultured for 10 days in the absence or presence of 5 μM DAPT. Representative dot plots of the different subpopulations of 3 independent experiments are shown.
Influence of the inhibition of Notch signaling with DAPT on development in hybrid human-mouse FTOC seeded with human CD34+CD1+ thymic progenitor cells. Murine fetal thymic lobes were seeded with human CD34+CD1+ thymic progenitor cells cultured for 10 days in the absence or presence of 5 μM DAPT. Representative dot plots of the different subpopulations of 3 independent experiments are shown.
Influence of inhibition of Notch signaling with DAPT on development in hybrid human-mouse FTOC seeded with human CD4+ISP thymic progenitor cells. Murine fetal thymic lobes were seeded with human CD4+ISP thymic progenitor cells cultured for 10 days in the absence or presence of 5 μM DAPT. Representative dot plots of the different subpopulations of 3 independent experiments are shown.
Influence of inhibition of Notch signaling with DAPT on development in hybrid human-mouse FTOC seeded with human CD4+ISP thymic progenitor cells. Murine fetal thymic lobes were seeded with human CD4+ISP thymic progenitor cells cultured for 10 days in the absence or presence of 5 μM DAPT. Representative dot plots of the different subpopulations of 3 independent experiments are shown.
We have now identified the lineage decision of CD34+ precursor cells as an important site of Notch action in human thymocyte development, wherein DAPT arrests T-cell development, similar to findings in mice.24,41 This is accompanied by a dramatic increase in B-cell number and identifies Notch transmembrane receptors as key players in determining the fate of lymphoid precursor cells in the human thymus.
Notch receptor signaling is important at several stages of hematopoiesis—HSC generation and self-renewal, T-cell commitment, B-cell development, and myeloid differentiation.42-45 When CD34+Lin- CB cells, a cell population highly enriched for HSCs, were cultured in FTOC, we found a dose-dependent decrease in CD34+ cells with 2 and 5 μM DAPT. This suggests that Notch signaling favors the self-renewal of HSCs. However, because we did not address by detailed phenotypic and functional analyses whether those CD34+ cells were HSCs, we were unable to conclude whether in the thymic microenvironment Notch signaling favors self-renewal and T-cell differentiation or favors only the latter. The observation that FTOC is a time-limited and exhaustive culture argues that HSC self-renewal is not a predominant property of Notch signaling. In contrast, CD34+Lin- CB cells cultured on Delta-like-1-expressing OP-9 stromal cells were maintained during the first week of culture19 (and M.D.S., unpublished observations, May 2003). It remains to be established whether factors that are differentially expressed in thymus stroma and OP-9-DL1 cells are responsible for HSC self-renewal or whether HSC self-renewal is a matter of different levels of Notch signaling. Furthermore, the increase in the number of dendritic cells and CD14+ cells in DAPT-treated cultures after 10 days of culture (data not shown) suggests that the differentiation of CD34+ cells toward other lineages may contribute to the decrease in CD34+ progenitor cells in DAPT-treated cultures.
Despite the continuous presence of the mouse thymic microenvironment, we observed the differentiation of CD34+ CB progenitor cells in DAPT-treated FTOC into cells with a B-cell phenotype expressing CD19 and HLA-DR. The cells were positive for CD79a and can thus be considered cells in the late pro-B stage. Similar cells are generated when CB CD34+CD38low progenitor cells are cocultured with the murine stromal cell line MS-5.46 Taken together, we conclude that Notch signaling represses the B-cell fate in early precursor cells in the thymus.
Importantly, CD34+CD1- thymic progenitor cells failed to develop into B lymphocytes under the same conditions. These data suggest that the B-cell-lineage potential of hematopoietic progenitor cells is lost immediately after entry into thymus. One study47 has shown that murine thymic CD117+CD44+CD25- (DN1) cells give rise to B lymphocytes when cultured on control OP-9 stromal cells. They observed less than 1% B-cell progenitors in the DN1 thymic cell population. They also observed that low levels of Notch signaling inhibit B-cell potential and that high levels induce T lymphopoiesis. Porritt et al48 have found that among DN1 prothymocytes, a subset can be characterized with B-lineage potential. This could indicate that cells with B-lineage potential have lost their B-cell potential before seeding the thymus by low levels of Notch signaling and that sustained Notch signaling in the thymus leads to T-lineage commitment. It is possible that FTOC in the presence of DAPT is not sensitive enough to detect a low precursor frequency of B cells or that human thymic CD34+CD1- cells have a lower frequency of B-cell precursors than murine DN1 cells. More detailed analysis of thymic CD34+ cells and culture on OP-9 stromal cells is required to determine the B-cell-lineage potential of these cells.
Our data indicate that Notch signaling also reduces NK cell differentiation in a thymic microenvironment. A direct effect of altered Notch signaling is compatible with the observation that increasing DAPT concentrations progressively inhibited T-cell maturation in FTOC and, at the same time, led to increased NK cell numbers. These data strongly suggest that the inhibition of Notch signaling is responsible for directing lymphoid progenitors into NK cells. These findings are also consistent with those from experiments in mice47 and in rats.34
It is clear that CD7 expression was influenced by the inhibition of Notch signaling. When CD34+Lin- CB cells were cultured in FTOC, both the frequency and the absolute numbers of CD7+ cells were significantly lower in DAPT-treated cultures in a dose-dependent way at the end of the culture. Given that after shorter incubation (10 days) the CD34+ cells did not express CD7 in the presence of DAPT (data not shown), we concluded that CD7 expression was inhibited from the start. This is compatible with the view that the progression of multipotent CD34+ progenitors toward T-/NK-cell progenitors is dependent on Notch signaling. However, CD7 expression was differentially regulated on T and NK cells because CD7 was down-regulated in developing T cells, whereas it was not affected in NK cells. This is best illustrated when thymic CD34+CD1- progenitors were cultured in FTOC at low DAPT concentrations. In these conditions, differentiation toward DP thymocytes occurred and was paradoxically enhanced, but CD7 expression level was decreased on T cells and unchanged on NK cells. This precludes the hypothesis that CD7 is directly influenced by Notch signaling and suggests that this occurs in the context of other factors that are present according to the cell type. Even though CD7 is expressed in early T-cell ontogeny, the roles that CD7 plays in T-cell development remain unclear. It has been shown in mice that the number of thymocytes and the induced thymocyte apoptosis is normal in CD7-deficient mice, which indicates that CD7 is not involved in the early stages of T-cell development.49 It was also demonstrated that NK-cell development and function are not impaired in CD7 disrupted mice.50
The function of Notch depends on the cell context and the activity of the Notch pathway itself. It is now known that Notch activity and the quantitative differences in the amount of Notch signaling influence cell fate.51 Given that, we had to inhibit Notch signaling strongly in CD34+ CB progenitors to arrest T-cell development and to obtain B lymphocytes. A more moderate reduction (2 μM DAPT) allowed the progenitor cells to develop into aberrant CD4ISP and DP cells without intracellular TCR-β. When CD34+CD1+ precursor cells were used, high concentrations of DAPT (10 μM) resulted in cell death whereas a lower concentration sufficed to enhance the development of aberrant DP thymocytes.
Molecular analysis revealed that the lack of TCRB chain expression resulted from the absence of VDJ rearrangement, whereas DJ rearrangement still occurred. Similarly, Wolfer et al14 have shown that thymocytes of mice with a conditional Notch1 deletion differentiate until the DN3 stage, though they lack VDJ rearrangement. Wolfer et al14 interpreted these findings in view of the potential role of Notch in apoptosis and proposed that defective apoptosis allows the generation and the survival of aberrant cells that normally should have undergone apoptosis. However, because Notch is known to inhibit signaling, it is also possible that the absence of Notch triggering bypasses pre-T-cell complex signaling with the resultant inhibition of VDJ recombination. Inhibition of the VDJ recombination normally occurs in cells after the pre-TCR complex is generated, and it is an important mechanism to mediate allelic exclusion. A similar mechanism has been demonstrated in 2 studies52,53 showing that transgenesis of an active cytosolic protein kinase D suppressed TCRB VDJ rearrangements by premature signaling. It is challenging to consider that Notch signaling acts not only as a T-cell driver but that it modulates signaling intensity to prevent inappropriate premature signaling in developing T cells. This could explain why no full T-cell differentiation toward TCRαβ thymocytes is observed with ICN-transduced human CD34+ progenitor cells in FTOC.17,18 The Notch signal that is delivered can be too strong and inhibits signaling from the pre-TCR complex.
We conclude that in human lymphoid development, B cells are preferentially generated in the absence of Notch, NK cells are generated in the presence of low amounts of Notch, and T cells require high levels of Notch signals. Notch is also required during later steps of T-cell differentiation to allow the generation of thymocytes with productive rearrangement of the TCRβ chain. The precise molecular mechanisms and their interplay with other signaling cascades remain to be established.
Prepublished online as Blood First Edition Paper, July 19, 2005; DOI 10.1182/blood-2005-02-0496.
Supported by grants from Concerted Research Action of the University of Ghent (01G00905) and Fund for Scientific Research-Flanders (G.0331.03).
M.D.S. and I.H. contributed equally to this manuscript.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr J. Unkeless (Mount Sinai School of Medicine, New York, NY) for his kind gift of the clone 2.4.G.2. We thank G. De Smet and I. Van de Walle for excellent technical assistance, C. De Boever for artwork, C. Collier and P. Scheire for animal care.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal