Abstract
The purpose of this study was to investigate treatment (tmt) with Rituximab ® in patients (pts) with ITP who had previously received R. Re-treatment (re-tmt) was administered either at standard (std) dose (375 mg/m2 x 4) n=15 or as augmented R, n= 14 (below). Eligibility criteria included primary chronic ITP, platelet count (plt ct)< 30K x 2 within a month, age > 12 years, and prior receipt of std dose R.
Augmented R pts were randomised to receive either R-CVP (group A,n=7) or Double Dose R (DDR) (group B,n=7). Group A pts received 1 dose of std dose R 1st and then 3 doses of R-CVP consisting of R, IV doses of Cyclophosphamide 750 mg/m2 & vincristine 1.4 mg/m2 (max 2 mg) and 100 mg prednisone PO daily for 5 days at 3 week intervals. Group B pts were treated with R 750 mg/m2/dose IV weekly for 4 weeks. Pts could receive IVIG during the initial tmt period. Two heavily pre-treated pts did not tolerate R-CVP well and refused the 3rd cycle; 1 std dose pt had an allergic reaction and could not complete her 4th infusion of re-tmt. Otherwise tmt was well tolerated. All pts had decline in circulating CD 19 positive B cells to < 0.03 X 109 and there were no significant decreases in IgG or IgM levels.
The table below summarises the response to the various tmts with R.
Overview of Response to Initial and Re-treatment with Rituximab
| Patient . | 1st Treatment . | 2nd Treatment . | DDR/R-CVP ♣ . | |||
|---|---|---|---|---|---|---|
| . | Median Plt. ct . | Response . | Median Plt Ct . | Response . | Median Plt. Ct . | Response . |
| ♣ 1 | 270 | CR | 319 | CR | 338 | CR |
| 2 | 102 | CR | 199 | CR | ||
| ♣ 3 | 157 | CR | 9 | NR | 24 | NR |
| 4 | 50 | PR | 34 | PR | 52 | PR |
| 5 ♣ | 50 | MR | 20 | MR | ||
| ♣ 6 | 65 | PR | 25 | PR | ||
| 7 | 8 | NR | 25 | NR | ||
| 8 | 7 | NR | 15 | NR | ||
| ♣ 9 | 10 | NR | 10 | NR | ||
| 10 | 6 | NR | 10 | NR | ||
| 11 | 195 | CR | 165 | CR | ||
| 12 | 325 | CR | 285 | CR | 267 | CR |
| ♣ 13 | 180 | CR | 10 | NR | ||
| 14 | 21 | NR | 169 | CR | ||
| 15 | 290 | CR | 150 | CR | ||
| 16 | 219 | CR | 155 | CR | ||
| 17 | 157 | CR | 80 | PR | ||
| 18 | 540 | CR | 350 | CR | ||
| 19 | 63 | PR | 13 | NR | ||
| 20 | 245 | CR | 30 | NR | ||
| 21 | 58 | PR | 181 | CR | ||
| 22 | 72 | PR | 72 | PR | ||
| 23 | 88 | PR | 78 | PR | ||
| 24 | 450 | CR | 636 | CR | ||
| Patient . | 1st Treatment . | 2nd Treatment . | DDR/R-CVP ♣ . | |||
|---|---|---|---|---|---|---|
| . | Median Plt. ct . | Response . | Median Plt Ct . | Response . | Median Plt. Ct . | Response . |
| ♣ 1 | 270 | CR | 319 | CR | 338 | CR |
| 2 | 102 | CR | 199 | CR | ||
| ♣ 3 | 157 | CR | 9 | NR | 24 | NR |
| 4 | 50 | PR | 34 | PR | 52 | PR |
| 5 ♣ | 50 | MR | 20 | MR | ||
| ♣ 6 | 65 | PR | 25 | PR | ||
| 7 | 8 | NR | 25 | NR | ||
| 8 | 7 | NR | 15 | NR | ||
| ♣ 9 | 10 | NR | 10 | NR | ||
| 10 | 6 | NR | 10 | NR | ||
| 11 | 195 | CR | 165 | CR | ||
| 12 | 325 | CR | 285 | CR | 267 | CR |
| ♣ 13 | 180 | CR | 10 | NR | ||
| 14 | 21 | NR | 169 | CR | ||
| 15 | 290 | CR | 150 | CR | ||
| 16 | 219 | CR | 155 | CR | ||
| 17 | 157 | CR | 80 | PR | ||
| 18 | 540 | CR | 350 | CR | ||
| 19 | 63 | PR | 13 | NR | ||
| 20 | 245 | CR | 30 | NR | ||
| 21 | 58 | PR | 181 | CR | ||
| 22 | 72 | PR | 72 | PR | ||
| 23 | 88 | PR | 78 | PR | ||
| 24 | 450 | CR | 636 | CR | ||
Among 15 pts on re-tmt with std dose R after ITP relapse, there were 7 complete responses (CR’s) (plt ct > 150,000/ul [150k] for 2 consecutive weeks) and 3 partial responses (PR’s) (plt ct between 50–150k for 2 consecutive weeks). Response to the 1st and 2nd std dose tmt courses was similar (n=15) but 3 pts (#3, 17 & 20 {see table}) with initial CR’s had limited to absent responses to re-tmt. No HAMA or HACA were detected in the 3 pts.
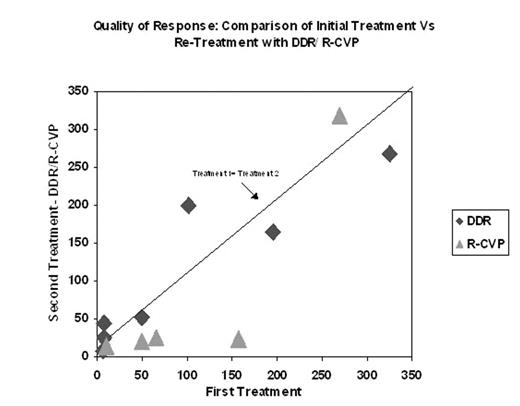
With augmented R, there were 3 CR’s among 7 pts randomized to the DDR group (#2, 11, 12). All 3 pts also had CR’s with the 1st tmt. Among 7 pts randomized to the R-CVP group, 1 pt had CR (also had CR with 1st R), 1 had PR (#6) and 4 pts failed to respond altogether; 1 pt just started R-CVP. No pt who did not respond to initial std dose R, responded to either DDR or R-CVP (see figure). 4 pts received re-tmt with std dose R and then augmented R. At this time it does not seem that augmented R has provided additional benefit to these 4 or the other retreated pts.
In conclusion, Rituximab re-tmt at std or double dose is well tolerated and generally produces similar response durations to initial R tmt in pts with relapsed chronic ITP. However 3/15 CR pts had no response to re-tmt. Augmented R, either as R-CVP or as DDR, did not provide any clear benefit to pts not responding initially to std R but may be superior to std dose R in some pts. R-CVP was less well tolerated than other regimens.
Quality of Response: Comparision of Initial Treatment Vs Re-Treatment with DDR R-CVP
Quality of Response: Comparision of Initial Treatment Vs Re-Treatment with DDR R-CVP
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal