Abstract
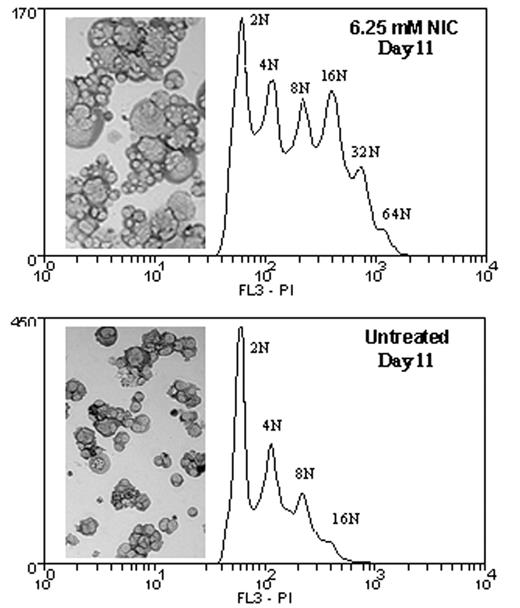
Megakaryocyte (Mk) maturation includes the development of polyploid cells via endomitosis. In vitro models of Mk differentiation can be used to gain a better understanding of the molecular mechanisms controlling this process. However, it is challenging to achieve ploidy levels in cultured human cells that are as high as those observed in vivo. Others have recently reported the use of chemical inhibitors to increase Mk ploidy (Lannutti et al., Blood 105:3875, 2005). Here, we show that nicotinamide (NIC), a form of vitamin B3, enhances the normal process of Mk polyploidization and leads to both a greater fraction of high ploidy cells and a greater degree of polyploidization. Human mobilized peripheral blood CD34+ cells were cultured in serum-free medium supplemented with thrombopoietin (TPO) to induce Mk differentiation. Beginning on day 5 of culture, cells were treated with nicotinamide (3 and 6.25 mM) and monitored for DNA content, growth, apoptosis, and surface marker expression. NIC treatment resulted in a greater fraction of Mks with high ploidy (DNA content greater than or equal to 8N). The ploidy of NIC treated cells continued to increase over the duration of the 13-day culture, whereas the ploidy of untreated cells peaked at day 9. On day 13 (8 days of NIC exposure), the percentages of high ploidy Mks for the untreated, 3 mM NIC, and 6.25 mM NIC conditions were 23%, 48%, and 63%, respectively. Furthermore, cells treated with NIC reached ploidy levels of 64N and 32N for 6.25 and 3 mM NIC, respectively, compared to 16N for untreated cells. NIC-treated cells also displayed dramatic differences in morphology - characterized by an increase in cell size, the presence of a more highly lobated nucleus, and an increased frequency of proplatelet-forming cells. Nicotinamide is known to inhibit poly(ADP-ribose) polymerase (PARP) and Sir2, which are both NAD+ dependent enzymes. Preliminary experiments show that PARP activity is low in cultured Mks and is not affected by addition of 6.25 mM NIC. Continued exposure (beginning at day 5) to the PARP inhibitors (and nicotinamide analogs) 3-aminobenzamide (3-AB) and benzamide at concentrations of 1, 3, and 6.25 mM was toxic to cells in a dose dependent manner. Interestingly, high doses of NIC (25 and 50 mM) were also toxic to cells. Remarkably, while Mk polyploidization and apoptosis are typically correlated, the increase in DNA content observed for NIC-treated cells occurred without significantly affecting the percentage of apoptotic Mks (assessed by Annexin V staining). These data suggest that it may be possible to partially decouple Mk apoptosis and polyploidization. Furthermore, while 6.25 mM NIC inhibited cell proliferation by ~35%, total expansion of cells cultured with 3 mM NIC was similar to that of untreated cells. This, combined with similar Mk commitment, as defined by a similar percentage of CD41+ cells, resulted in a greater overall number of high ploidy Mks in cultures treated with NIC. Since there is a direct correlation between Mk DNA content and platelet production (Mattia et al., Blood 99:888, 2002), these results suggest a possible therapeutic benefit of NIC for the management of thrombocytopenia. Similarly, NIC could also be used as an additive to ex vivo Mk cultures destined for transplantation.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal