Abstract
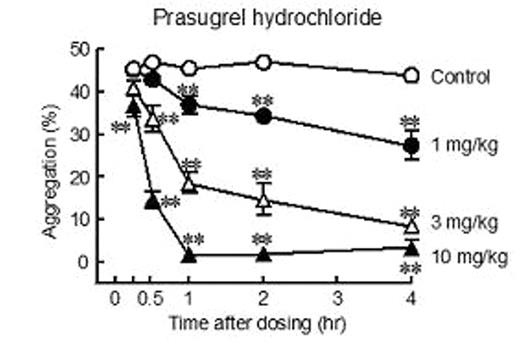
The thienopyridine antiplatelet prodrug clopidogrel bisulfate (C) is widely used for atherothrombotic diseases. However, several reports have described a limitation of C, namely, poor platelet inhibition in many patients following oral dosing. Prasugrel (hydrochloride), currently under investigation in a Phase 3 trial in acute coronary syndrome patients undergoing percutaneous coronary intervention, is a novel thienopyridine antiplatelet prodrug. In recent clinical studies, prasugrel has shown more consistent platelet inhibition than C. To date, several preclinical studies have demonstrated that the prasugrel base formulation has more potent antiplatelet and antithrombotic effects than C. The effects of prasugrel hydrochloride (P), which has a higher solubility than the base formulation, have not been reported. The present studies examined the effects of P on platelet aggregation, arterial thrombosis, and bleeding time in rats. Single oral administration of P (0.3 to 3 mg/kg) resulted in inhibition of platelet aggregation (IPA) induced by ADP in a dose- and time-related manner with an ED50 value of 1.1 mg/kg at 4 hr postdose. After dosing of 3 and 10 mg/kg P, statistically significant IPA was observed at 30 and 15 min, and the time required to achieve 50% IPA was approximately 50 and 23 min, respectively (Figure). These results indicate that P has potent antiplatelet effects with an extremely fast onset of action. Multiple P dosing also resulted in potent IPA, with an ED50 value of 0.45 mg/kg/day (p.o.) at 4 hr after the last dose. Our previous report indicated an ED50 value of C at 4 hr after single oral dosing of 16 mg/kg (
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal