Abstract
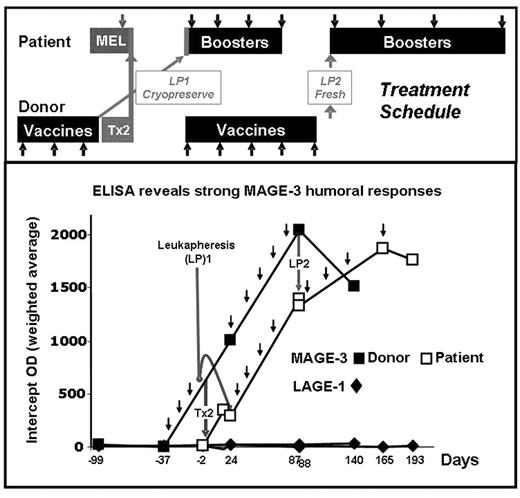
A MAGE-3 positive, stage IIIA MM patient with a twin donor provided a unique opportunity to study if vaccination with MAGE-3 protein induces immune responses in the healthy twin, and if these responses can be adoptively transferred to the patient and expanded by booster vaccines. The patient was treated with MEL 200 tandem Tx (Tx1: auto, Tx2: syngeneic) and MAGE-3 recombinant protein in ASO2B adjuvant. The MAGE-3 negative, identical twin donor received 3 vaccines and was leukapheresed (LP1) one week after the 3rd vaccine to store vaccine-induced immune cells. Donor G-CSF mobilized stem cells (Tx2) were transfused fresh to the patient after MEL conditioning. Donor LP1 was transfused to the patient 21 days post Tx2 and followed by 8 booster vaccines. The donor continued vaccinations and LP2 was collected one week after the 8th vaccine and transfused to the patient who had received 4 of 8 planned booster vaccines. Ab responses to MAGE-3 and controls NY-ESO-1, LAGE-1, MAGE-1, MAGE-4, and p-53 were assessed by ELISA. MAGE-3 specific CD8+ T-cell responses to autologous EBV transformed cells pulsed with pooled 20-mer overlapping peptides from MAGE-3 were assessed by ELISPOT. Neither twin had pre-existing anti-MAGE-3 humoral immunity. The donor twin developed MAGE-3 ab up to 1/6400. The patient had a detectable anti-MAGE-3 titer post donor Tx2 (1/400) which was boosted by subsequent vaccinations (1/1600). After the transfer of LP2 and vaccines 5–8, the patient had MAGE-3 ab titers similar to those in the donor. There was low but significant cross-reactivity only to MAGE-4. A vaccine-induced CD8+ cellular response to a previously undescribed MAGE-3115–123, HLA-A68 restricted epitope was observed in the donor and patient. MAGE-3115–123 is in the same region as (but is distinct from) two other known HLA-A2 and -B40 epitopes. Further studies with recombinant MAGE-3 protein and tetramers are in progress to study if this epitope is indeed naturally processed and presented. The patient is in CR nearly 1 yr post Tx2. We show for the first time that vaccinating a healthy donor with a defined cancer-testis antigen can induce both cellular and humoral responses and that such responses can be transferred and expanded post Tx in the recipient. Close inspection of the ab curves shows that humoral immunity was transferred with the donor stem cells (Tx2) and expanded in the patient by further booster vaccines. We could not establish with certainty that LP1 and LP2 contributed further to transferring humoral or cellular immunity. It is hoped that this approach may be used to induce a specific anti-MM immune response after autologous or allogeneic Tx and reduce MM relapse.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal