Abstract
Background: Patients with SMM/IMM are at high risk for progression to active MM and are appropriate candidates for chemoprevention trials. High IL-1beta levels are a useful surrogate marker for progression from SMM/IMM to active myeloma.
Methods: We carried out a Phase II clinical trial of IL-1Ra (Anakinra) in patients with SMM/IMM to determine the biologic activity, progression-free rate, and toxicity of IL-1Ra. Since IL-1beta induces paracrine IL-6, we hypothesized that IL-1Ra would inhibit IL-6 production and myeloma cell growth. Patients that had ≥ 10% bone marrow plasma cells and/or an IgG or IgA M-spike ≥ 3 g/dL and did not require immediate chemotherapy were eligible. Patients received 100 mg of Anakinra (IL-1Ra) SQ qd for a total duration of 6 months. Non-progressors were allowed to continue on therapy with IL-1Ra until they converted to active myeloma. IL-1beta levels were measured in a bioassay with a read out of IL-1 inducible IL-6 production. CRP levels served as a surrogate for IL-6 production.
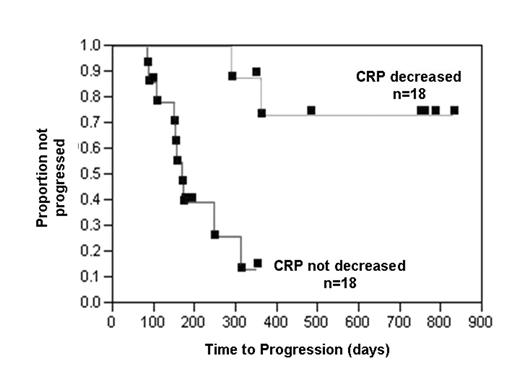
Results: Thirty-six patients are included for analysis based on intent to treat at diagnosis. At baseline, twenty-nine patients had elevated functional IL-1beta levels consistent with progressive disease and 12 patients had radiologic evidence of disease on bone survey. The median TTP for the entire group was 1 year and 2 patients exhibited a minor response based on M-protein reduction. A proportional hazards analysis was performed and the variables examined were: % bone marrow plasma cells, plasma cell labeling index (PCLI), circulating plasma cells, albumin, M-protein level, IgA subtype, CRP, CRP % reduction ≥ one-third, beta-2 microglobulin, creatinine, calcium, hemoglobin, urine total protein, presence of lytic bone disease, and IL-1 level. Univariate Cox model results showed that % BMPC, PCLI, circulating plasma cells, CRP % reduction, presence of lytic disease, and IL-1 level were all statistically significant (p < .05) variables. However, only the CRP % reduction from baseline remained significant in the multivariate analysis (p < 0.01). The median TTP for patients without (n=18) and with (n=18) a decrease in CRP was 6 months and > 2 years, respectively (p < .0001). Toxicities included injection site reactions during the first month of therapy and asymptomatic neutropenia necessitating a reduction in therapy to every other day. Five patients in whom the CRP decreased and the baseline PCLI was > 0 also demonstrated a parallel decrease in the PCLI. Several of the patients with progressive disease on IL-1Ra alone could later be rescued with the addition of low dose dexamethasone (20 mg once a week) suggesting that the endogenous IL-1beta levels were too high to inhibit with IL-1Ra alone. Conclusion: In summary, IL-1Ra can prolong the TTP to active myeloma in responsive SMM/IMM patients through inhibition of IL-6 production (decrease in CRP) and myeloma cell growth.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal