Abstract
Iron overload, a potentially serious consequence of multiple blood transfusions, can be effectively managed with chelation therapy. Deferasirox, an investigational once-daily oral chelator, has been evaluated in a 1 year study of iron-overloaded adult and pediatric patients (n=184) with transfusion-dependent anemia including β-thalassemia, myelodysplastic syndromes (MDS) and Diamond-Blackfan anemia (DBA). Patients were stratified into four daily dose groups (5, 10, 20 and 30 mg/kg) according to baseline liver iron concentration (LIC; 2–3, >3–7, >7–14 and >14 mg Fe/g dw, respectively). Iron balance was determined for all patients, based on transfusional iron intake and chelator-induced iron excretion, derived from the change in LIC during the study (Table 1).
Patient characteristics, LIC, serum ferritin and iron excretion/intake ratio during deferasirox treatment
| . | β-thalassemia (n=85) . | DBA (n=30) . | MDS (n=47) . | Other anemias (n=22) . |
|---|---|---|---|---|
| *Mean ± SD | ||||
| Age*, years | 24.7 ± 10.0 | 16.1 ± 10.3 | 65.1 ± 12.5 | 35.8 ± 22.9 |
| Body weight, kg | 51.1 ± 14.1 | 39.1 ± 18.7 | 70.4 ± 12.5 | 56.1 ± 18.5 |
| Deferasirox dose*, mg/kg | 23.8 ± 7.2 | 23.6 ± 7.4 | 20.0 ± 8.3 | 21.9 ± 6.5 |
| Iron intake*, mg/kg/day | 0.35 ± 0.12 | 0.40 ± 0.11 | 0.28 ± 0.14 | 0.31 ± 0.19 |
| <0.3, n (%) | 28 (33) | 6 (20) | 25 (53) | 10 (45) |
| 0.3–0.5, n (%) | 49 (58) | 19 (63) | 20 (43) | 7 (32) |
| >0.5, n (%) | 8 (9) | 5 (17) | 2 (4) | 5 (23) |
| Serum ferritin*, ng/mL | ||||
| Baseline | 4321 ± 2881 | 3245 ± 2439 | 3343 ± 1978 | 3144 ± 1850 |
| Absolute change | −386 ± 1626 | −118 ± 1373 | −268 ± 2053 | −750 ± 1517 |
| LIC*, mg Fe/g dw | (n=76) | (n=26) | (n=28) | (n=17) |
| Baseline | 19.3 ± 10.9 | 18.8 ± 10.7 | 15.6 ± 11.9 | 15.1 ± 6.2 |
| Absolute change | −4.7 ± 8.6 | −1.6 ± 6.5 | −5.7 ± 6.3 | −3.7 ± 6.3 |
| Iron excretion/intake ratio | 1.5 ± 0.90 | 1.1 ± 0.46 | 1.7 ± 0.93 | 1.6 ± 1.48 |
| . | β-thalassemia (n=85) . | DBA (n=30) . | MDS (n=47) . | Other anemias (n=22) . |
|---|---|---|---|---|
| *Mean ± SD | ||||
| Age*, years | 24.7 ± 10.0 | 16.1 ± 10.3 | 65.1 ± 12.5 | 35.8 ± 22.9 |
| Body weight, kg | 51.1 ± 14.1 | 39.1 ± 18.7 | 70.4 ± 12.5 | 56.1 ± 18.5 |
| Deferasirox dose*, mg/kg | 23.8 ± 7.2 | 23.6 ± 7.4 | 20.0 ± 8.3 | 21.9 ± 6.5 |
| Iron intake*, mg/kg/day | 0.35 ± 0.12 | 0.40 ± 0.11 | 0.28 ± 0.14 | 0.31 ± 0.19 |
| <0.3, n (%) | 28 (33) | 6 (20) | 25 (53) | 10 (45) |
| 0.3–0.5, n (%) | 49 (58) | 19 (63) | 20 (43) | 7 (32) |
| >0.5, n (%) | 8 (9) | 5 (17) | 2 (4) | 5 (23) |
| Serum ferritin*, ng/mL | ||||
| Baseline | 4321 ± 2881 | 3245 ± 2439 | 3343 ± 1978 | 3144 ± 1850 |
| Absolute change | −386 ± 1626 | −118 ± 1373 | −268 ± 2053 | −750 ± 1517 |
| LIC*, mg Fe/g dw | (n=76) | (n=26) | (n=28) | (n=17) |
| Baseline | 19.3 ± 10.9 | 18.8 ± 10.7 | 15.6 ± 11.9 | 15.1 ± 6.2 |
| Absolute change | −4.7 ± 8.6 | −1.6 ± 6.5 | −5.7 ± 6.3 | −3.7 ± 6.3 |
| Iron excretion/intake ratio | 1.5 ± 0.90 | 1.1 ± 0.46 | 1.7 ± 0.93 | 1.6 ± 1.48 |
Transfusion requirements and iron intake during the study varied widely between diseases. However, LIC and serum ferritin decreases were consistently achieved in all patient groups. More than one-third (38%) of patients, most of whom had MDS or other anemias, had an iron intake rate <0.3 mg/kg/day (average: 0.2 mg/kg/day; corresponding to 5.6 ml RBC/kg/month). In these patients, deferasirox at 10 and 20 mg/kg reduced LIC.
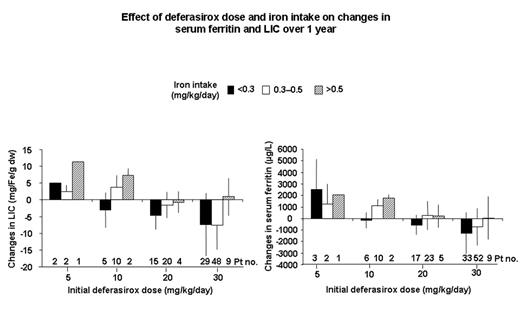
Overall, an iron intake- and dose-related response pattern was observed for both LIC and serum ferritin (Figure 1).
Effect of deferasirox dose and iron intake on changes in serum ferritin and LIC over 1 year
Effect of deferasirox dose and iron intake on changes in serum ferritin and LIC over 1 year
According to these results, deferasirox demonstrates the ability to stabilize and effectively decrease body iron levels at doses of 10, 20 and 30 mg/kg/day, depending on the degree of iron intake. In conclusion, dosing of chelation therapy should be guided by a patient’s transfusion requirements and the treatment goal, which is either to maintain or reduce body iron.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal