Abstract
Background: There is a continuing need for safe, effective and convenient therapies for prophylaxis of venous thromboembolism (VTE) after major orthopedic surgery. LY517717 (LY), an oral direct inhibitor of activated factor X (fXa), has been well tolerated in healthy subjects and is being developed to address these needs.
Objective: To assess the safety and efficacy of LY and to determine whether one or more doses of LY is non-inferior to enoxaparin in preventing VTE in patients undergoing total knee replacement (TKR) or total hip replacement (THR).
Methods: In a double-blind, double-dummy, dose-escalation study, patients undergoing TKR or THR were randomized to receive one of six oral doses of LY (25, 50, 75, 100, 125 or 150mg) or enoxaparin, 40 mg SC once daily. LY was administered fasting 6–8 hours after wound closure and then each morning. Enoxaparin was administered the night before surgery and then every evening. Both treatments were continued for a total of 6–10 doses. Patients underwent mandatory bilateral venography within 12 hours of the last dose of oral study drug and were assessed for symptomatic deep vein thrombosis (DVT), pulmonary embolism (PE) and bleeding events through day 30 (±7). All VTE and bleeding events were adjudicated by a blinded independent central adjudication committee. DVT detected by mandatory venography and symptomatic DVT and PE events were included in the combined VTE endpoint. Non-inferiority would be declared if the upper limit of the 90% confidence interval (CI) for the absolute difference in VTE incidence between an LY dose and enoxaparin was less than 0.14.
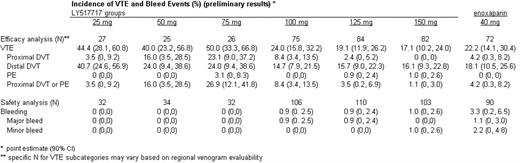
Results: 511 subjects were randomized. 507 patients received at least one dose of LY or enoxaparin and were included in the safety analyses. 391 patients had an evaluable bilateral venogram or experienced an objectively confirmed clinical VTE and were included in the efficacy analyses. The 3 lowest doses were stopped early due to lack of efficacy, and the study was completed with the 3 highest doses. VTE and bleed event data through the treatment and follow-up periods are summarized in the table. The 100, 125 and 150 mg doses of LY were non-inferior to enoxaparin in the incidence of symptomatic or venographic DVT or PE (upper limits for the 90% CI of the absolute differences are 0.13, 0.05 and 0.01, respectively). Adjudicated bleed events were uncommon in both LY and enoxaparin patients.
Conclusion: The 100, 125 and 150 mg doses of LY517717 were as efficacious as enoxaparin 40 mg for the prevention of VTE following TKR or THR in this study. LY was safe and well tolerated by study patients, with no significant difference in bleeding risk compared with enoxaparin.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal