Abstract
We have recently reported that human platelet factor 4 (PF4; CXCL4) reverts the anergic CD4+CD25+ T regulatory (Tr) cells phenotype, and impairs their suppressive activity in vitro, suggesting a previously unrecognized role of PF4 in the regulation of immune responsiveness (
Liu, et al. J Immunol 174:2680–86, 2005
). How PF4 modulates CD4+CD25+ Tr cell-mediated suppression and proliferation remains to be determined. PF4, a heparin binding CXC chemokine normally found in platelet alpha granules, is secreted upon platelet activation. Neuropilin-1 (NRP1) has been recently identified as a specific surface marker for CD4+CD25+ Tr cells. NRP1, a receptor involved in axon guidance, angiogenesis, and the activation of T cells, is constitutively expressed on the surface of CD4+CD25+ Tr cells independently of their activation status (Bruder, et al. Eur.J Immunol 34:623–30, 2004
). NRP1 binds semaphorin 3A and participate in the establishment of cellular contacts between naïve T cells and dendritic cells (formation of the “immunological synapse”) and therefore is essential for the initiation of the primary immune response. Very recently, it has also been reported that NRP1 may interact with heparin-binding proteins (i.e. fibroblast growth factor, FGF) other than the heparin-binding form of vascular endothelial growth factor (VEGF165) (West, et al. J Biol Chem. 280:13457–64, 2005
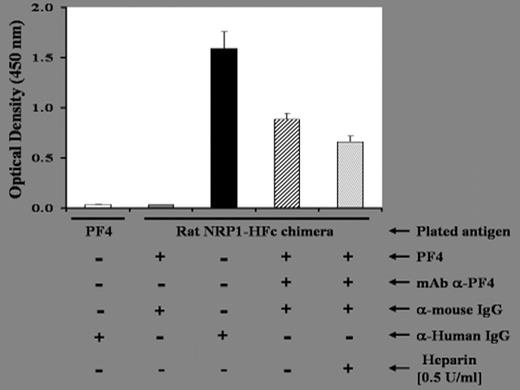
). We postulate that NRP1 acts as a receptor or co-receptor for PF4. To address this hypothesis, we first investigated the interaction of PF4 with NRP1 by ELISA, then we isolated CD4+CD25+ Tr cells from normal donor’s peripheral blood mononuclear cells by positive selection on magnetic beads (Miltenyi Biotec, Auburn, CA), to perform Immunoprecipitation, Western Blotting, Real Time PCR and Flow Cytometry studies of CD4+CD25+ Tr cells cultured in the absence or the presence of PF4. We report that in CD4+CD25+ Tr cells: 1) Direct binding between PF4 and recombinant rat neuropilin-1/Human-Fc chimera (the amino acid sequence of rat NRP1 is 98% identical to human; R&D Systems) can be detected in ELISA and it is only slightly affected by the presence of heparin (Figure 1); 2) As detected by immunoprecipitation and Western Blotting, by using anti-NRP1 (Santa Cruz Biotechnology and Miltenyi Biotec) and/or anti PF4 mAbs that we generated, NRP1 co-precipitates with PF4; 3) As detected by real-time PCR, NRP1 gene expression, in CD4+CD25+ Tr cells, is enhanced in the presence of PF4 (four fold vs. medium only); 4) As detected by flow cytometry using the anti-BDCA-4 mAb (specific for human NRP1; Miltenyi Biotec), NRP1 surface expression, on CD4+CD25+ Tr cells, is enhanced in the presence of PF4 (three fold vs. medium only). While a spliced variant of CXCR3 has been reported to be a receptor for PF4 on endothelial cells, the antithetical effects of PF4 on CD4+CD25+ and CD4+CD25− T cells suggest that there are at least two different mechanisms and/or receptors involved in mediating PF4 effects on T cells. Our data suggest that neuropilin-1 is a potential new receptor for PF4. Further studies are needed to determine whether PF4 activates or suppresses NRP1-associated signaling pathways.2005, The American Society of Hematology
2005

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal