Abstract
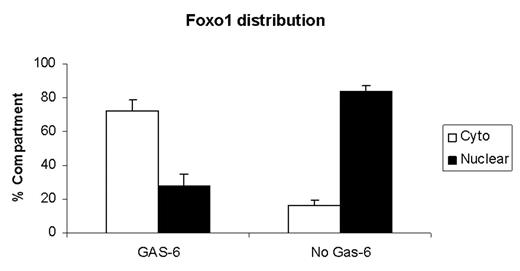
Growth Arrest Specific gene product 6 (GAS-6), a γ-carboxylated protein expressed in quiescent fibroblasts and endothelial cells, exerts an anti-apoptotic function by binding to the receptor tyrosine kinase Axl. Recently, our laboratory has demonstrated that gas6-Axl interactions activate PI3-kinase with subsequent Akt phosphorylation during gas6-mediated protection from apoptosis. The current study explores further the mechanism by which this survival mechanism is achieved. FOXO1 is a member of the Forkhead family of transcription factors that plays a role in the expression of pro-apoptototic genes. Phosphorylation of FOXO1 at Thr24, Ser256 and Ser319 results in phospho-FOXO1 translocation from the nucleus to the cytoplasm, with consequent suppression of FOXO1 transcriptional activity and inhibition of apoptosis. In the present study we show, for the first time, that the treatment of serum-starved endothelial cells with 100 ng/ml of GAS-6 induces FOXO1 phosphorylation in a time dependent manner. Phosphorylated FOXO1 is translocated from the nucleus to the cytoplasm as evidenced by Western blot analysis of both nuclear and cytoplasmic extracts. Using fluorescence microscopy, FOXO1 is found predominantly in the nucleus during apoptosis induced by serum starvation. Upon gas6 stimulation, phosphorylated FOXO1 is translocated to the cytoplasm (see Figure 1). It is suggested that anti-apoptotic genes are then released from suppression and are thereby able to mediate cell survival. Both FOXO1 phosphorylation and translocation are suppressed by Wortmannin, a PI3-kinase inhibitor demonstrating that FOXO1 phosphorylation is PI3-kinase dependent. These results provide mechanistic insight of how gas6 rescues endothelial cells from serum-starvation-induced apoptosis.
Foxo1 distribution
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal