Abstract
The chemotherapeutic agent 5-azacytidine (5-AZA) is an active agent in the treatment of myelodysplastic syndrome (MDS). Phase III data with 5-AZA for MDS of all types suggest a response rate (CR+PR) of 23% and hematologic improvement rate of 37% (
Methods: Retrospective analysis of outcomes in 13 patients (median age 67; range 50–82) with CMML, who were drawn from a group of 165 consecutive patients treated with 5-AZA between 1991 and 2004 at UCSF on an NCI Special Exception Program, was performed. All patients signed informed consent to participate in the Expanded Access Protocol and IRB approval at UCSF was obtained to retrospectively review this data. IPSS of patients were 2 LOW, 7 INT-1, 3 INT-2, and 1 HIGH. 11 patients had CMML-1 and 2 patients had CMML-2. Responses were determined using the MDS International Working Group (
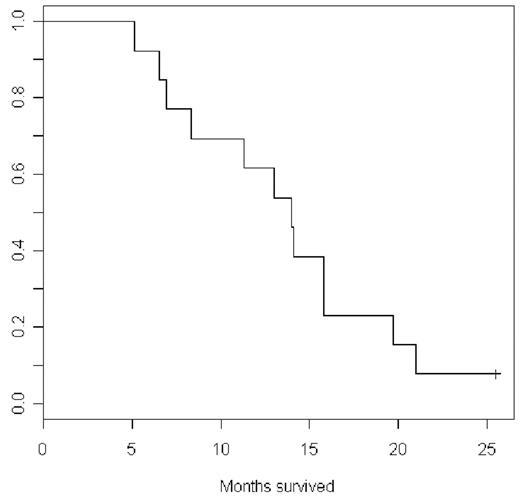
Results: Three patients (23%) had CR and 6 patients (46%) had HI at 4 months. 9/13 patients had a response at 4 months for an overall response rate of 69% (95% CI 38.6% to 90.0%). Cytogenetic responses were seen in 1 out of 4 evaluable patients. The median AML free survival was 11 months. The median overall survival was 14 months.
Conclusions: For patients with CMML, 5-AZA provides an effective treatment option with significant improvements in peripheral blood counts, marrow blasts, and transfusion requirements. The overall response rate of 69% correlates well with larger studies in MDS.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal