Abstract
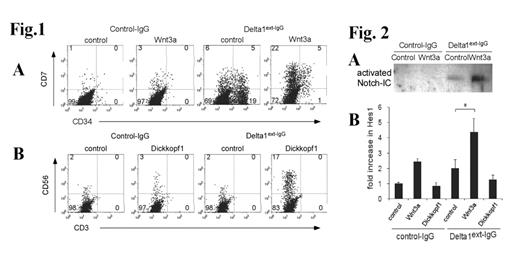
The Wnt and Notch signaling pathways have critical roles in cell fate decisions. However, the interaction of these pathways is poorly understood. Using highly purified Wnt3a and immobilized Notch ligand, Delta1ext-IgG, we investigated the mechanisms involved in Wnt and Notch signaling interactions during hematopoietic stem cell differentiation. When CD34+CD38- cord blood stem cells were cultured for 2 to 3 weeks with five growth factors (SCF 300ng/ml, Flt-3L 300ng/ml, TPO 100ng/ml, IL-6 100ng/ml, and IL-3 10ng/ml), most precursor cells lost CD34 expression and differentiated into mature cells, most of which were monocytes. However, as we previously reported, when cells were cultured with Delta1ext-IgG, we found an increased percentage of lymphoid progenitors (CD34+CD7+) and more mature lymphoid precursors (CD34-CD7+) in the cell population. The addition of purified Wnt3a (100ng/ml) alone without Delta1ext-IgG did not significantly change the percentage of CD7+ cells (1% vs. 3%). However, when both Wnt3a and Delta1ext-IgG were added, we saw an increased percentage of CD7+ cells (11% with Delta1ext-IgG alone to 27% with both) (Fig. 1A). Wnt3a also enhanced gene expression of CD3ε and preTα induced by Delta1ext-IgG. These results suggest that Wnt3a enhances the effect of Notch signaling on T-cell lineage development. To test the role of endogenous Wnt signaling, we added Dickkopf1, an inhibitor of Wnt signaling. When CD34+CD38- cells were cultured with Dickkopf1 (300ng/ml) alone for a 2 week, the percentage and number of CD56+ NK cells was unaffected. However, when cultured with Dickkopf1 and Delta1ext-IgG, the percentage and number of CD56+ NK precursor cells were increased (2% with Delta1ext-IgG alone vs. 17% with Dickkopf1 and Delta1ext-IgG; 0.2 ×105 vs. 1.5×105, p<0.01) (Fig. 1B). This result shows that decreased endogenous Wnt signaling enhances the generation of NK cells in the presence of Notch signaling. To address whether Wnt signaling affects Notch signaling by modulating protein levels, we assessed the amount of activated Notch1 intracellular domain with or without Wnt 3a by Western blot (Fig. 2A). After stimulation for 24 h, Wnt3a increased the amount of activated Notch1 intracellular domain induced by Delta1ext-IgG. Wnt3a also enhanced the expression of a primary target gene of Notch signaling, Hes1, determined by quantitative RT-PCR (Fig. 2B). In contrast, Dickkopf1 reduced Delta1ext-IgG-induced Hes1 expression. These results suggest that Wnt signaling directly modulates Notch signaling. Thus, these studies suggest that Wnt signaling is a key factor in cell fate determination at the point of NK/T cell commitment that is mediated via an interaction with Notch signaling. These studies also suggest that Wnt directly regulates Notch signaling by modulating protein turnover.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal