Abstract
Introduction: INTERCEPT plasma (I-FFP) for transfusion is prepared with a photochemical treatment (PCT) system using amotosalen (S-59) and long-wavelength UVA light to inactivate a broad spectrum of blood-borne pathogens. For Phase 3 clinical trials, 6 US blood centers prepared an inventory of ~10,000 I-FFP units by processing ~250 mL whole blood-derived (WB) or apheresis (APH) plasma units using a prototype PCT system. In these trials, I-FFP effectively supported patients with congenital and acquired coagulopathies or TTP. The prototype PCT system has been modified to treat up to 635 mL of plasma in a single PCT process, yielding up to three ~200 mL doses while maintaining pathogen inactivation efficacy. This modified PCT system intended for commercialization was evaluated in process validation studies in 3 European blood centers under routine operating conditions. After processing with the commercial PCT system, the effect on coagulation factor activity and retention was assessed in APH plasma (
Methods: Whole blood and/or APH plasma units were collected at 3 European blood centers. Three-unit pools (~600 mL) of WB plasma were prepared. APH plasma (~600 mL) was collected using Autopheresis C (Baxter) or MCS+(Haemonetics) devices. Blood bank personnel processed a total of 60 WB plasma pools and 90 APH plasma units using the commercial PCT system. Baseline and I-FFP plasma samples were collected, frozen below -60°C, and sent to Cerus for assay of factors I (fibrinogen), II, V, VII, VIII, IX, X, XI, and XIII, proteins C (PC) and S (PS), and antithrombin III (AT). Alpha-2 antiplasmin (AP) was assayed by a reference laboratory. Comparative data from a representative subset of I-FFP units prepared for the Phase 3 trials using the prototype PCT system were obtained from samples collected during PCT processing and stored at ≤−70°C. Retention of activity is expressed as the proportion (%) of pre-treatment (baseline) activity remaining after PCT.
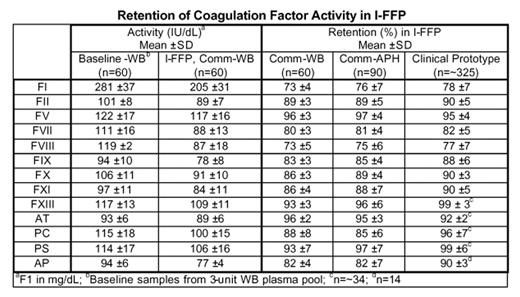
Results: Retention of coagulation factor activity in WB and APH I-FFP prepared with the commercial PCT system (Comm) was 73–76% of baseline fibrinogen and FVIII activity, and 80–97% of baseline for factors II, V, VII, IX, X, XI, XIII, PC, PS, AT, and AP (Table). Retention of activity in I-FFP prepared with the commercial PCT system was similar to that of I-FFP prepared with the clinical prototype.
Conclusion: The PCT system intended for commercialization provides multiple I-FFP doses with a single PCT process. Retention of coagulation factor activity in WB and APH plasma processed with the commercial PCT system was similar to that of I-FFP used in Phase 3 trials to effectively support patients with congenital and acquired coagulopathies or TTP.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal