Comment on Sato et al, page 428
Successful invasion and remodeling of the uterine vasculature by extravillous trophoblasts (EVTs) are crucial steps in early pregnancy. Sato and colleagues provide evidence that platelet-derived factors, particularly chemokines, play an important role in this process.
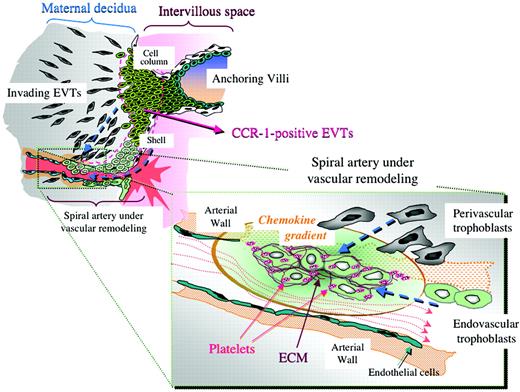
The fetus' anticipated demand for sufficient blood flow requires the remodeling of the maternal spiral arteries in early pregnancy. This is accomplished through the invasion of EVTs into the uterine wall and its blood vessels. The EVTs displace smooth muscle and endothelial cells, resulting in blood vessels with a larger diameter, increased blood flow, and reduced resistance. If this process does not occur properly, disorders such as preeclampsia, fetal loss, and growth retardation may occur.1 However, despite its importance in pregnancy, the mechanism behind EVT migration to the maternal arteries remains unknown.
Previously, Sato and colleagues2 demonstrated that the CC chemokine receptor 1 (CCR1) is expressed on trophoblasts and that its ligands promote EVT migration. Along with other studies indicating that human platelets release several chemoattractants, including chemokines,3 this finding suggested that platelet-derived chemoattractants might play an important role in EVT invasion and differentiation. In this issue of Blood, Sato et al examine this hypothesis and provide evidence to support a physiologic role of platelet-derived chemoattractants on EVT invasion and differentiation.
Through immunohistochemistry on human placental tissues from early pregnancy, maternal platelets and extracellular matrices (ECMs) were detected among endovascular trophoblasts within the lumen of maternal spiral arteries. Since ECM is known to trigger platelet activation, it is likely that these platelets have been activated and have released bioactive substances, such as chemokines, that may support trophoblastic vascular infiltration. This hypothesis is further supported by the presence of P-selectin, a protein that is transported to the cell surface during platelet activation, colocalizing with CD41 on the cell surface.
Using an in vitro Matrigel invasion assay, Sato and colleagues found that maternal platelets have many short- and long-term effects on the invasive properties of EVTs. Coculturing EVTs with platelets, but not with peripheral blood mononuclear cells (PBMCs), enhanced their invasion. In addition, platelet-conditioned medium (CM) had an enhancing effect on EVT invasion in a dose-dependent manner. When treated with an anti-CCR1 neutralizing antibody, EVT migration was significantly reduced, suggesting that the chemokine-CCR1 system plays a crucial role in EVT migration to the maternal spiral arteries. Platelet-derived soluble factors also appear important in the differentiation of EVTs to the endovascular phenotype. However, antibody treatment suggested that the processes are controlled by different platelet-soluble factors, as differentiation of EVTs was not inhibited by the suppression of the chemokine-CCR1 system.FIG1
Possible chemokine gradient produced by platelets in spiral arteries and its estimated effects on trophoblast infiltration toward the arteries. See the complete article beginning on page 428.
Possible chemokine gradient produced by platelets in spiral arteries and its estimated effects on trophoblast infiltration toward the arteries. See the complete article beginning on page 428.
Since CCR1 neutralization did not completely inhibit EVT migration, platelet-derived soluble factors other than chemokines most likely also play a role in EVT migration. Though other studies have suggested that a lipid mediator, sphingosine-1-phosphate (S1P), is released from platelets,4 Sato and colleagues found that lipid removal from platelet-CM by charcoal stripping did not affect migration. However, heat inactivation of peptides halted migratory activity, indicating that protein factors, such as chemokines, are the premier factors in promotion of EVT migration.
These findings suggest that platelet activation and release of chemoattractants, triggered by contact with the ECM of invading EVTs, set off a positive feedback loop that promotes further EVT infiltration to maternal spiral arteries. Abundant expression on trophoblasts of thrombomodulin and tissue- and urokinase-type tissue plasminogen activators may ensure that abnormal coagulation does not occur during this process. Greater understanding of the reciprocal regulation between platelets and EVTs may help develop more targeted therapies for preeclampsia, pregnancy loss, and fetal growth retardation. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal