Comment on Hebeis et al, page 635
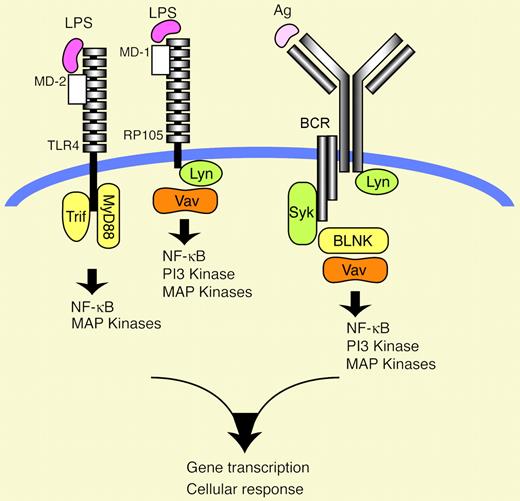
Emerging evidence indicates that the Vav family of signaling molecules plays a critical role in LPS receptor signaling, as well as in B-cell–receptor (BCR) signaling.
B lymphocytes must integrate 2 distinct signals before they proliferate and make antibodies; the first coming from antigens and the second coming from either helper T cells or microbial products such as lipopolysaccharide (LPS). The involvement of Vav1 and Vav2 in B-cell–receptor (BCR) signaling was first suggested by biochemical findings that these proteins were tyrosine phosphorylated in response to BCR ligation. Vav1 and Vav2 form protein complexes, by means of their Src homology 2 (SH2) domain, with the spleen tyrosine kinase (Syk), the coreceptor CD19, and the adaptor molecule B-cell linker protein (BLNK, also known as SH2-containing leukocyte protein of 65 kDa [SLP-65]) in activated B cells. These interactions are important in regulating cellular localization of Vav1/Vav2, undergoing tyrosine phosphorylation, and thereby enhancing their guanine nucleotide exchange factor (GEF) activity. Although there was a brief period when it was suggested that Vav1 was a GEF for Ras, it is now accepted that Vav family proteins are not directly involved in Ras regulation, but rather target the Rho family guanosine triphosphatases (GTPases) Rac1 and Rac2.1
The validity of the idea that Vav1 and Vav2 are indeed important players in BCR signaling was reinforced by subsequent mouse genetic ablation experiments; loss of both Vav1 and Vav2 resulted in greatly compromised BCR signaling capability and were probably responsible for a block in humoral immune responses as well as B-cell maturation.2,3 The importance of Vav1 and Vav2 in antigen receptor signaling is clear, but 2 lines of recent observations suggested the additional possibility that Vav family proteins could participate in innate receptor signaling as well. First, not only BCR- but also LPS-dependent proliferation was profoundly inhibited in Vav1–/–Vav2–/– double knock-out B cells.2 Second, introduction of dominant interfering mutants of Vav1 into transformed macrophages inhibited cytosine-phosphate-guanosine (CpG) DNA-mediated up-regulation of nuclear factor κB (NF-κB) activity and tumor necrosis factor α (TNF-α) secretion.4 In this issue of Blood, Turner and colleagues, by comparing LPS receptor signal ing between wild-type and Vav1–/–Vav2–/– primary B cells, provide strong evidence that Vav1 and Vav2 are required for LPS-mediated Akt (one of the PI3K [phosphatidylinositol 3-kinase] targets) and NF-κB activation, thereby contributing to B-cell survival and proliferation.FIG1
How Vav is activated in RP105 signaling is unclear, but the scenario that Lyn phosphorylates Vav, thereby enhancing its GEF activity, could be envisaged by the following: (1) activation of Lyn by RP105 stimulation; and (2) similar downstream defects in RP105 signaling between Lyn–/– and Vav1–/–Vav2–/– B cells.5
How Vav is activated in RP105 signaling is unclear, but the scenario that Lyn phosphorylates Vav, thereby enhancing its GEF activity, could be envisaged by the following: (1) activation of Lyn by RP105 stimulation; and (2) similar downstream defects in RP105 signaling between Lyn–/– and Vav1–/–Vav2–/– B cells.5
As the LPS recognition unit on the B-cell surface, there are 2 types of receptors: Toll-like receptor 4 (TLR4)/MD2 and RP105 (also called CD180)/MD1. The extracellular domains of TLR4 and RP105 associate with MD2 and MD1, respectively, to form heterodimers, thereby forming the binding sites to LPS (see figure).6 Since loss of one of these 4 molecules (TLR4, MD2, RP105, MD1) leads to decreased responses to LPS, these 2 recognition systems are thought to function at least partly in a nonredundant manner for the full activation of LPS-mediated responses in B cells. In terms of dissection of the 2 systems, the specific antibody for RP105 stimulation is available, but the lack of such a reagent for TLR4 stimulation hampers detection of TLR4-specific signaling. Hence, Turner and colleagues looked at RP105 signaling more in detail and carefully. What they found was that the RP105 system indeed used Vav1 and Vav2 to activate Akt and NF-κB, although Vav1 was more dominant. This was not due to indirect effects such as differences in RP105 expression levels or in B lymphocyte populations between wild-type and Vav1–/–Vav2–/– knock-out mice. Given the general importance of Akt and NF-κB pathways in cell survival and proliferation, these defects likely account for the reduced RP105-mediated survival and proliferation observed in Vav1–/–Vav2–/– double knock-out B cells. Moreover, such a cellular basis in Vav1–/–Vav2–/– B cells could help explain why these knock-out mice fail to mount sufficient antibodies in response to a hapten-LPS conjugate. RP105–/– mice are also incapable of producing antibodies in response to this conjugate, further supporting this explanation.7
This study unravels an as-yet unappreciated role of the Vav family proteins in LPS receptor signaling, particularly in RP105 signaling, thereby implying that Vav proteins function as an integrator for 2 signals emanating from antigens and microbial products. However, key questions remain to be addressed: Do Vav proteins participate in TLR4 signaling, in addition to RP105 signaling? How is Vav activated in RP105 signaling? Does it require tyrosine phosphorylation and GEF activity, as in the case of BCR signaling? More important, how do Vav proteins coordinate BCR and LPS signals, thereby influencing downstream signaling? Answers to these and other questions will undoubtedly add to a more complete picture of B-cell activation and differentiation. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal