Abstract
Recently, inhibitory cytokine pathways for leukocyte chemoattraction and activation have been identified, but there is little insight into the operational mechanisms except for models that rely on simple receptor antagonism. We have previously identified the existence of a murine eosinophil inhibitory pathway mediated by the CXC chemokine ligand 9 (CXCL9, Mig [monokine induced by interferon-γ]) that impressively blocks eosinophil chemoattraction and function, but the mechanism has remained elusive. We now demonstrate that Mig's inhibitory action extends beyond receptor antagonism alone. Notably, in addition to inhibiting eotaxin-induced filamentous actin (F-actin) formation and chemoattraction, Mig potently blocks platelet activating factor (PAF)– and leukotriene B4 (LTB4)–induced responses. Remarkably, Mig-treated eosinophils display an abnormal F-actin assembly in the absence of agonist stimulation. Additionally, Mig pretreatment inhibits eotaxin-induced activation of the Rho–guanosine triphosphatase (GTPase) Rac, and Rac2-deficient eosinophils demonstrate an impaired transmigration and actin polymerization response to eotaxin stimulation. Furthermore, Mig was unable to inhibit eotaxin-induced responses in Rac2-deficient eosinophils. Finally, using CCR3 gene–targeted cells, Mig's inhibitory activity is demonstrated to be mediated by CC chemokine receptor 3 (CCR3). Thus, by altering agonist-induced signaling and abrogating cytoskeletal reorganization by a Rac2-dependent mechanism, Mig markedly inhibits eosinophil responses to diverse stimuli. These results establish evidence that distinct chemokines can use CCR3 to induce opposing signals in eosinophils.
Introduction
Chemokines are the largest family of cytokines in the human genome and have been shown to have an essential role in diverse processes including inflammation, organ development, tumor cell growth and metastasis, atherosclerosis, and angiogenesis.1-4 In the first phase of studies in the chemokine field, most work was focused on identifying individual ligands and receptors. Indeed, more than 45 chemokines and 16 7-transmembrane–spanning G-protein–coupled receptors (most being promiscuous) have been characterized. In the second phase of research, attention was focused on understanding the individual and cooperative interaction of individual chemokines and their receptors. More recently, attention has focused on the role of naturally occurring chemokine antagonists; at least 8 different chemokines that act as natural antagonists have been described.5-10 In addition to being agonists for their cognate receptors, these chemokines induce inhibitory activity by acting as competitive antagonists for a variety of other receptors. For example, while monocyte chemoattractant protein 3 (MCP-3) is normally a ligand for CC chemokine receptor 2 (CCR2), it is a competitive antagonist for CCR5.5 Indeed, numerous microbial pathogens have exploited chemokine receptors by encoding for chemokine homologs that have antagonistic properties.11,12 Initial mechanistic analysis has focused primarily on receptor blockade, but for several of these natural antagonists the pronounced inhibitory activity cannot be explained by competitive receptor binding alone.13-16 Surprisingly, there is little insight into the mechanisms that regulate the inhibitory activity of natural chemokines except for models that rely on simple receptor antagonism. Only recently has it been proposed that diverse signaling events can be induced by ligands that bind to the same receptor.17
Eosinophil accumulation in tissues is a characteristic feature of several important medical disorders including atopic diseases, parasitic infections, and hypereosinophilic syndromes.18,19 Inappropriate accumulation and activation of eosinophils can result in the release of a range of inflammatory mediators, including toxic granule proteins, cytokines, and activated oxygen species, that contribute to disease pathogenesis.20 Focusing on the asthmatic lung, the recruitment of eosinophils is regulated by integrated signaling events among chemokines that operate through chemokine receptor 3 (CCR3), lipid mediators (eg, platelet activating factor [PAF] and leukotrienes), enzymes (eg, acidic mammalian chitinase), and the T helper 2 (Th2) cytokines interleukin-4 (IL-4), IL-5, and IL-13.21-23 IL-5 is critical for the induction of peripheral blood eosinophilia, and also acts in synergy with the eosinophil-specific chemokines, eotaxin-1 and eotaxin-2 (CCR3 ligands), induced by IL-4 and IL-13, to coordinate tissue accumulation of eosinophils. Following binding to CCR3, the eotaxins activate numerous events including actin reorganization, calcium mobilization, and production of reactive oxygen species.24,25 In addition, the eotaxins couple to signaling pathways that have been demonstrated to mediate migration, including the mitogen-activated protein kinase family and the Rho family of small guanosine triphosphatases (GTPases).26-28 In neutrophils, Rho GTPases (eg, Rho, Rac, and Cdc42 [cell division cycle 42]) control a wide spectrum of cellular functions, including cytoskeletal organization, transcription, superoxide production, and cell growth and proliferation; however, their role in eosinophils has been addressed only in studies demonstrating the critical participation of Rac2 in regulating respiratory burst function29,30 ; their role in chemotaxis has not been defined.31-34
Recently, we and others have identified the existence of an eosinophil inhibitory pathway mediated by the CXC chemokine ligand 9 (CXCL9, Mig [monokine induced by interferon-γ]), which significantly blocks eosinophil chemoattraction and function in vitro and in vivo.13,14,35 For example, a dosage as low as 1 μg/mouse blocks approximately 90% of pulmonary eosinophilia induced by eotaxin-2. To our knowledge, this is the first eosinophil inhibitory chemoattraction pathway mediated by a natural protein; accordingly, the existence of this pathway has considerable potential therapeutic significance, as the mechanistic pathway may contain a number of targets for drug intervention. However, to date, it has not been determined if Mig's inhibitory pathway blocks only signaling induced by eotaxins or if it blocks signaling induced by non-CCR3 ligands as well. This distinction is important since the latter is considerably more attractive from a therapeutic standpoint. Additionally, the mechanism of Mig's inhibitory activity on eosinophils has not been elucidated. The importance of elucidating novel pathways that regulate eosinophil trafficking to the lung has been recently reinforced by the dramatic protection from experimental asthma seen in 2 different eosinophil-deficient mouse lines.36,37 Lee et al targeted the destruction or depletion of eosinophils by using an eosinophil-specific promoter to drive expression of a cytocidal protein diphtheria toxin A.36 Allergen challenge of these mice demonstrated that eosinophils were required for pulmonary mucus accumulation and the airway hyperresponsiveness associated with asthma. In comparison, Yu et al developed mice harboring a deletion of a high-affinity GATA binding site in the GATA1 promoter (Δdbl-GATA), which presumably leads to the specific ablation of the eosinophil lineage.38 Induction of experimental asthma in Δdbl-GATA mice demonstrated that eosinophils contribute substantially to airway remodeling, supporting an important role for eosinophils in chronic asthma.
Here we have determined the mechanism of negative signaling induced by Mig in eosinophils. We first show that in addition to inhibiting eotaxin-induced filamentous actin (F-actin) formation and chemoattraction, Mig potently blocks PAF- and leukotriene B4 (LTB4)–induced responses, providing strong evidence that Mig's inhibitory activity may be mediated by mechanisms other than CCR3 receptor antagonism alone. Additionally, Mig-treated eosinophils were shown to display an abnormal F-actin assembly in the absence of agonist stimulation. However, Mig pretreatment inhibited eotaxin-induced activation of the Rho GTPase Rac. We also demonstrate that Rac2–/– eosinophils display a chemotactic and actin assembly defect similar to Mig-treated eosinophils, suggesting Rac2 as a critical downstream signaling molecule in this inhibitory pathway. Supporting this view, Mig was unable to inhibit eotaxin-induced responses in eosinophils in the absence of Rac2. Finally, using CCR3 gene–targeted cells, Mig's inhibitory activity is demonstrated to be mediated by CCR3. Thus, these results establish evidence that distinct chemokines can use CCR3 to induce opposing signals in eosinophils; as such, these findings have significant therapeutic ramifications.
Materials and methods
Mice
Male and female, 8- to 12-week-old, CD2–IL-5 transgenic mice (BALB/c) were housed under specific pathogen-free conditions and used as a source of eosinophils, as previously reported.39 The CD2–IL-5 transgenic mice were mated with CCR3 gene–targeted BALB/c mice to be used as a source for CCR3-deficient eosinophils.40 Similarly, Rac2-deficient eosinophils were derived from CD2–IL-5 transgenic Rac2-deficient mice.34
Chemotaxis assay
Chemotactic responses were determined by transmigration through respiratory epithelial cells as previously described.41 Leukocytes (1.5 × 106) in Hanks buffered salt solution (HBSS) + 0.5% bovine serum albumin (BSA, low endotoxin; Sigma, St Louis, MO) were placed in the upper chamber, and the chemoattractant (PAF at 10 nM and LTB4 at 1 nM in HBSS + 0.5% BSA) was placed in the lower chamber. Eosinophils were obtained by use of splenocytes from IL-5 transgenic mice where approximately 30% of the cells are eosinophils. In some experiments, eosinophils were purified (84%-92% purity) from the spleen by immunomagnetic negative selection, as previously described.39 Pretreated cells were incubated with chemokine (Mig or eotaxin-2) for 15 minutes at 37°C, and then washed twice to remove chemokine from the media. Transmigration was allowed to proceed for 3 hours.
Calcium mobilization
Purified eosinophils (1 × 106 cells/mL) were loaded with 5 μM Fura-2 AM (Molecular Probes, Eugene, OR) in HBSS/1% fetal bovine serum (FBS) and incubated at 37°C for 30 minutes in the dark. Cells were washed twice with flux buffer (145 mM NaCl, 4 mM KCl, 1 mM NaHPO4, 0.8 mM MgCl2, 1.8 mM CaCl2, 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], and 22 mM glucose) and resuspended at 1 × 106 cells/mL. Cells (2 mL) were prewarmed and stimulated in a cuvette with a continuously stirring magnetic bar at 35°C in a RatioMaster fluorometer (Photon Technology, South Brunswick, NJ). Data were recorded as the relative ratio of fluorescence emitted at 510 nm after excitation at 340 and 380 nm (y-axis) over time (x-axis).
Actin polymerization
Agonist-induced actin polymerization was assessed using nitrobenzoxadiazole (NBD)–phallacidin (Molecular Probes). Purified eosinophils were resuspended at 2.5 × 106 cells/mL in phosphate-buffered saline (PBS) and stimulated with eotaxin (10 nM), PAF (10 nM), Mig (200 nM), or the specified combination at 37°C for indicated amounts of time. Following stimulation, cells were fixed in 3.7% formaldehyde for 60 minutes. Lysophosphatidylcholine (100 mg/mL; Sigma) and 3.3 × 10–7 M NBDphallacidin were added to the cells and incubated for 1 hour in the dark. Cells were analyzed on a FACScalibur with a linear fluorescence channel (FL1) where the fluorescence is proportional to F-actin content. Relative F-actin content is expressed as the ratio of the mean channel fluorescence between chemokine- and media alone–stimulated cells. The percent inhibition of the maximal response is calculated for relative F-actin content for Mig-treated cells compared with untreated cells.
Actin localization
To characterize subcellular localization of F-actin, 2.5 × 104 cells were seeded onto chamber slides (Nalge Nunc International, Naperville, IL) in RPMI for 1 to 2 hours at 37°C to allow the cells to adhere to the slide. Eosinophils were then stimulated with 10 nM eotaxin-1 for 10 seconds, fixed with 2% paraformaldehyde (pH 7.4; Fisher Scientific, Hampton, NH) for 20 minutes, and then permeabilized with 0.1% Triton X-100 (Sigma). After incubation in 2% BSA for 15 minutes, the cells were stained with rhodamine-labeled phalloidin (Molecular Probes) for 1 hour at room temperature (RT) and then washed 3 times with PBS. Slides were mounted using SlowFade Light Antifade Kit with 4′6-diamidino-2-phenylindole (DAPI; Molecular Probes). Z series of fluorescence images were captured with a Leica DMIRB fluorescence microscope (Heidelberg, Germany) at 63 magnification with an ORCA-ERC4742-95 camera (Hamamatsu) equipped with a deconvolution system (Leica) driven by Openlab 3.1 software (Improvision, Lexington, MA).
Rac activation assay
Guanosine triphosphate (GTP)–bound Rac was precipitated with a p21-activated kinase (PAK)–p21 binding domain (PBD)–based assay (Upstate Biotechnology, Lake Placid, NY). Eosinophils (1 × 106) in 250 μL RPMI were stimulated with eotaxin-1 (10 nM) for the indicated times. Pretreated eosinophils were incubated with Mig (200 nM) for 2 minutes at 37°C prior to addition of agonist. Stimulation was stopped by addition of 5 mL ice-cold PBS. After centrifugation, cell pellets were resuspended in 0.5 mL magnesium-containing lysis buffer (MLB). Aliquots were saved for immunoblot analysis of total Rac. Lysates were rotated with 5 μg PAK-PBD agarose at 4°C for 60 minutes. The agarose pellet was washed twice with 1 × MLB and resuspended in 30 μL reducing sample buffer. Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 4%-12% Bis [bisphenol A dimethacrylate]–Tris [tris(hydroxymethyl)aminomethane] gels, MES [2-morpholinoethanesulfonic acid] buffer; Invitrogen, Frederick, MD), transferred to nitrocellulose membrane (Invitrogen), and blotted for pan Rac (BD Transduction Labs, San Diego, CA).
Chemokine binding
For Mig binding, wild-type and CCR3-deficient eosinophils (2 × 105) were incubated for 15 minutes at 4°C with 1 nM to 1000 nM murine Mig. Following chemokine exposure, cells were washed, fixed with 2% paraformaldehyde, washed, and then incubated with 500 ng (5 μg/mL) polyclonal goat anti–murine Mig (R&D Systems, Minneapolis, MN) or control goat immunoglobulin G (IgG) at 4°C for 15 minutes. Cells were stained with fluorescein isothiocyanate (FITC)–conjugated secondary antibody. For eotaxin-1 binding studies, eosinophils were incubated with a competitor chemokine (eg, Mig at 20, 200, 500, and 2000 nM) for 5 minutes on ice, prior to addition of biotinylated 20 nM eotaxin-1 (Sigma) or JE (R&D Systems) for 30 minutes on ice. Cells were stained with streptavidin–phycoerythrin (PE; BD Pharmingen, San Diego, CA) for 30 minutes on ice. Chemokine binding is reported as percent mean channel fluorescence over baseline (no chemokine or no competitor) mean channel fluorescence.
Results
Mig inhibits PAF- and LTB4-induced chemotaxis
In our prior studies, we have demonstrated that Mig directly blocks eosinophil responses to the eotaxin chemokines and the ability of IL-13 to induce pulmonary eosinophilia.14 One interpretation of this data would be that Mig was mediating its effect by functioning as a competitive antagonist of the eotaxin receptor CCR3. However, when eosinophils are pretreated with Mig, their subsequent chemotactic response to the non-CCR3 ligand PAF is strongly inhibited in a dose-dependent manner (Figure 1A). Following a 15-minute pretreatment with Mig at 1, 10, and 50 nM, eosinophil transmigration toward PAF (10 nM) was reduced 40%, 68%, and 82%, respectively (Figure 1A). The mean decrease in PAF (10 nM)–induced eosinophil responses following Mig (50 nM) pretreatment was 75% plus or minus 7.3% (n = 4 experiments). As a control, the mean decrease in chemotactic response toward eotaxin-1 (1 nM) following Mig pretreatment was 91.5% plus or minus 4.2% (n = 4 experiments). In order to determine if the effect of Mig was applicable to other non-CCR3 ligands, we examined the ability of Mig pretreatment to inhibit LTB4-induced transmigration. Following Mig pretreatment at 10, 50, and 200 nM, eosinophil transmigration toward LTB4 (1 nM) was reduced 57%, 75%, and 84%, respectively (Figure 1B). In contrast, Mig (50 nM) pretreatment had no effect on PAF- or LTB4-induced neutrophil responses (17 908 ± 4467 vs 17 981 ± 3775 transmigrated neutrophils for PAF and 7413 ± 681 vs 6919 ± 591 for LTB4; n = 2 experiments). These data demonstrate that Mig has strong and specific eosinophil inhibitory activity toward both CCR3 and non-CCR3 chemoattractants, and suggest a more complex mechanism than receptor blockade alone.
Mig inhibits eosinophil transmigration toward the non-CCR3 ligands PAF and LTB4 in vitro. Eosinophil transmigration following pretreatment with buffer or Mig is shown. Data represent mean ± SD of eosinophils that migrated toward PAF 10 nM (A) or LTB4 1 nM (B). *P < .05.
Mig inhibits eosinophil transmigration toward the non-CCR3 ligands PAF and LTB4 in vitro. Eosinophil transmigration following pretreatment with buffer or Mig is shown. Data represent mean ± SD of eosinophils that migrated toward PAF 10 nM (A) or LTB4 1 nM (B). *P < .05.
Mig inhibits agonist-induced actin polymerization
Following exposure to a chemoattractant, eosinophils undergo a series of events, including reorganization of actin filaments and subsequent rapid shape changes, culminating in chemotaxis.42,43 We were first interested in examining the effect of Mig on agonist-induced actin polymerization in eosinophils. Accordingly, F-actin formation was measured kinetically following exposure to either eotaxin-1 alone, Mig alone, or eotaxin-1 and Mig together. Eotaxin-1 (10 nM) induced a rapid and transient polymerization of actin molecules (Figure 2A). There was a transient increase in F-actin content of 57% plus or minus 12% (P = .001, n = 3 experiments) within 10 seconds of eotaxin-1 exposure. In the presence of Mig (40 nM), eotaxin-1–induced F-actin formation was reduced (P = .02, Figure 2A). Notably, Mig inhibited eotaxin-1–induced actin polymerization in a dose-dependent manner; at 10 seconds, the optimal time point chosen for subsequent studies, F-actin content was inhibited 31% plus or minus 2% and 52% plus or minus 6% (P = .02, n = 3 experiments) in the presence of increasing concentrations of Mig (20 nM and 40 nM, respectively). Similarly, in the presence of Mig (200 nM), eotaxin-2 (10 nM)–induced actin polymerization was reduced by 51% plus or minus 4% (n = 2 experiments). Notably, treatment of eosinophils with Mig alone (2-200 nM) did not induce F-actin formation; the modest increase in F-actin content seen at 30 seconds (Figure 2A) was not reproducibly detected (data not shown). Furthermore, since Mig inhibited transmigration toward a non-CCR3 ligand, we hypothesized that Mig would also inhibit actin polymerization in response to PAF. To test this, F-actin content was measured following exposure to PAF (10 nM) alone and to Mig (2, 20, and 200 nM) and PAF together. Mig inhibited PAF-induced actin polymerization in a dose-dependent manner (Figure 2B).
Mig inhibits agonist-induced actin polymerization. (A) Eosinophils were treated with 10 nM eotaxin-1 (♦), 40 nM Mig (▪ and dashed line), or 40 nM Mig and 10 nM eotaxin-1 (▴) for the indicated period of time. Cells were fixed and stained with NBD-phallacidin. Relative F-actin content is expressed as the ratio of the mean channel fluorescence between eotaxin- and media alone–stimulated cells. A representative experiment is shown (n = 3). (B) Mean (± SD) percent inhibition of 10 nM PAF-induced F-actin polymerization in eosinophils in the presence of 2 to 200 nM Mig (n = 3 experiments). The analysis was performed following 10 seconds of chemokine exposure. *P < .05. (C) Actin localization was determined by fluorescence microscopy. Cells were fixed and stained with rhodamine-labeled phalloidin after stimulation for 10 seconds with buffer alone (resting), eotaxin-1 (10 nM), Mig (200 nM), or eotaxin-1 and Mig. Images were acquired with a fluorescence microscope equipped with a deconvolution system driven by Openlab software. Results are representative of 4 experiments.
Mig inhibits agonist-induced actin polymerization. (A) Eosinophils were treated with 10 nM eotaxin-1 (♦), 40 nM Mig (▪ and dashed line), or 40 nM Mig and 10 nM eotaxin-1 (▴) for the indicated period of time. Cells were fixed and stained with NBD-phallacidin. Relative F-actin content is expressed as the ratio of the mean channel fluorescence between eotaxin- and media alone–stimulated cells. A representative experiment is shown (n = 3). (B) Mean (± SD) percent inhibition of 10 nM PAF-induced F-actin polymerization in eosinophils in the presence of 2 to 200 nM Mig (n = 3 experiments). The analysis was performed following 10 seconds of chemokine exposure. *P < .05. (C) Actin localization was determined by fluorescence microscopy. Cells were fixed and stained with rhodamine-labeled phalloidin after stimulation for 10 seconds with buffer alone (resting), eotaxin-1 (10 nM), Mig (200 nM), or eotaxin-1 and Mig. Images were acquired with a fluorescence microscope equipped with a deconvolution system driven by Openlab software. Results are representative of 4 experiments.
Mig regulates actin localization
To further examine the effect of Mig on cytoskeletal changes important in cell movement, we examined eotaxin-induced actin subcellular localization following Mig treatment. To optimize the analysis, we selected the maximum time point for eotaxin-induced changes in F-actin content (10 seconds) and a dose of 200 nM Mig. Cell spreading and considerable cortical F-actin assembly were seen in eosinophils stimulated with 10 nM eotaxin-1 (Figure 2C). In contrast, eotaxin-1–induced cortical F-actin polymerization was impaired in the presence of 200 nM Mig (Figure 2C). With Mig stimulation alone, the F-actin structure was markedly altered compared with untreated control or agonist-stimulated eosinophils (Figure 2C). The F-actin assembly was punctate in distribution in Mig-treated eosinophils; whereas, a perinuclear distribution was seen in control cells (Figure 2C). Collectively, these data demonstrate that Mig profoundly impairs the assembly and organization of the actin cytoskeleton.
Mig inhibits agonist-induced Rac activation
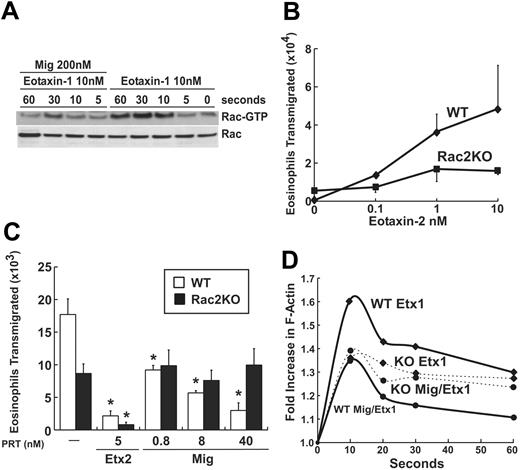
The Rho family of small GTPases has been shown to be an essential regulator of cell movement and cytoskeletal rearrangement in certain cell types.44 In neutrophils, 2 Rac members, Rac1 and Rac2, have been shown to direct neutrophil function, including F-actin generation, chemotaxis, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity.34,45-49 Surprisingly, the role of Rac2 in eosinophil chemoattraction has not been previously determined. We hypothesized that Mig would inhibit agonist-induced Rac activation in eosinophils. Using a PAK pull-down assay and an antibody that recognizes both Rac1 and Rac2, we examined the relative levels of Rac-GTP in lysates from eotaxin-1–stimulated eosinophils with and without Mig pretreatment. Whereas only a small amount of activated Rac was detected in unstimulated eosinophils, stimulation with eotaxin-1 induced formation of Rac-GTP within 10 seconds of stimulation, with maximal levels detected at 30 seconds (Figure 3A). Notably, Mig pretreatment resulted in reduced formation of Rac-GTP in response to eotaxin-1 stimulation (Figure 3A).
Rac2–/– eosinophils demonstrate impaired chemotaxis
Neutrophils from rac2 gene–targeted mice exhibit multiple functional defects, including F-actin generation and directed cell movement.34,46,48 It is also interesting to note that the abnormal actin localization in Mig-treated eosinophils resembled the localization seen in Rac2-deficient granulocytes (Figure 2C).48 As such, we hypothesized that Rac2-deficient eosinophils would display impaired chemotaxis and actin polymerization, similar to Mig-treated eosinophils. To test this hypothesis, we generated hypereosinophilic mice that were deficient in Rac2 and examined eotaxin-induced transmigration for wild-type and Rac2-deficient eosinophils. Wild-type eosinophils exhibited a strong chemotactic response to eotaxin-2, with a dose response seen between 0.1 to 10 nM (Figure 3B). Eotaxin-2–induced chemotaxis by Rac2-deficient eosinophils was reduced 45% to 70% when compared with wild-type eosinophils (Figure 3B). This deficiency was evident over the 100-fold concentration range for eotaxin-2. The mean decrease in eotaxin-2 (1 nM)–induced responses was 52% plus or minus 13% (n = 3 experiments). We next investigated the effects of Mig pretreatment on Rac2-deficient eosinophils. As expected, wild-type eosinophils were inhibited by Mig pretreatment in a dose-dependent manner (Figure 3C). In contrast, Mig pretreatment had no inhibitory effect on Rac2–/– eosinophils. It is important to note that Rac2–/– eosinophils had a blunted chemotactic response compared with wild-type eosinophils. As a positive control, we examined eosinophil inhibition by eotaxin-2 pretreatment. Notably, eotaxin-2 pretreatment desensitized eosinophils to eotaxin-2 (Figure 3C). However, in contrast to Mig, eotaxin-2–induced desensitization was independent of Rac2 (Figure 3C). For example, wild-type and Rac2–/– eosinophils were inhibited by 93% and 92%, respectively, with eotaxin-2 pretreatment.
Mig inhibits agonist-induced Rac activation and Rac2 is required for Mig's inhibitory activity. (A) Eosinophil lysates were used for affinity precipitation with 5 μg PAK-PBD for 60 minutes at 4°C. Active Rac-GTP precipitated by PAK-PBD was separated on SDS-PAGE, transferred to nitrocellulose membrane, and blotted for pan-Rac, followed by enhanced chemiluminescence (ECL) detection. In the bottom panel, aliquots of lysates were immunoblotted and probed for Rac to confirm equal protein expression. Representative blots are shown (n = 3). (B) Wild-type (WT, ♦) and Rac2-deficient (▪; KO indicates knock out) eosinophil transmigration toward eotaxin-2 is shown. Data represent mean ± SD of eosinophils that migrated toward eotaxin-2 (0-10 nM). A representative experiment is shown (n = 3). P = .03 between wild-type and Rac2–/– at 1 and 10 nM based on paired Student t test. (C) Wild-type (□) and Rac2-deficient (▪) eosinophil transmigration toward eotaxin-2 is shown following pretreatment with buffer, eotaxin-2 (Etx2; 5 nM), or Mig (0.8-40 nM). Data represent mean ± SD of eosinophils that migrated toward eotaxin-2 (1 nM). A representative experiment is shown (n = 3). *P < .05 when compared with pretreatment of buffer alone. (D) Wild-type (solid lines) or Rac2-deficient (dashed lines) eosinophils were treated with 12 nM eotaxin-1 (♦), or 40 nM Mig and 12 nM eotaxin-1 (•) for the indicated period of time. Cells were fixed and stained with NBD-phallacidin. Relative F-actin content is expressed as the ratio of the mean channel fluorescence between eotaxin- and media alone–stimulated cells.
Mig inhibits agonist-induced Rac activation and Rac2 is required for Mig's inhibitory activity. (A) Eosinophil lysates were used for affinity precipitation with 5 μg PAK-PBD for 60 minutes at 4°C. Active Rac-GTP precipitated by PAK-PBD was separated on SDS-PAGE, transferred to nitrocellulose membrane, and blotted for pan-Rac, followed by enhanced chemiluminescence (ECL) detection. In the bottom panel, aliquots of lysates were immunoblotted and probed for Rac to confirm equal protein expression. Representative blots are shown (n = 3). (B) Wild-type (WT, ♦) and Rac2-deficient (▪; KO indicates knock out) eosinophil transmigration toward eotaxin-2 is shown. Data represent mean ± SD of eosinophils that migrated toward eotaxin-2 (0-10 nM). A representative experiment is shown (n = 3). P = .03 between wild-type and Rac2–/– at 1 and 10 nM based on paired Student t test. (C) Wild-type (□) and Rac2-deficient (▪) eosinophil transmigration toward eotaxin-2 is shown following pretreatment with buffer, eotaxin-2 (Etx2; 5 nM), or Mig (0.8-40 nM). Data represent mean ± SD of eosinophils that migrated toward eotaxin-2 (1 nM). A representative experiment is shown (n = 3). *P < .05 when compared with pretreatment of buffer alone. (D) Wild-type (solid lines) or Rac2-deficient (dashed lines) eosinophils were treated with 12 nM eotaxin-1 (♦), or 40 nM Mig and 12 nM eotaxin-1 (•) for the indicated period of time. Cells were fixed and stained with NBD-phallacidin. Relative F-actin content is expressed as the ratio of the mean channel fluorescence between eotaxin- and media alone–stimulated cells.
Next, we examined the role of Rac2 in actin polymerization in eosinophils. Although transient increases in F-actin were observed with eotaxin-1 (12 nM) stimulation for 10 seconds in wild-type eosinophils, the generation of F-actin in Rac2-deficient eosinophils in response to eotaxin-1 was significantly reduced (Figure 3D). Notably, in the presence of Mig, eotaxin-induced F-actin formation was inhibited 40% in wild-type eosinophils, but not in Rac2-deficient eosinophils (Figure 3D). Collectively, these data suggest that Rac2 is required not only for normal agonist-induced chemotaxis and actin polymerization in wild-type eosinophils, but also for Mig's inhibitory activity mediated through regulation of actin assembly.
Stimulation of eosinophils with a chemoattractant results in increases in cytosolic calcium due to a combination of intracellular calcium release and an influx of extracellular calcium. The role of intracellular calcium transients in granulocyte actin polymerization and chemotaxis is unclear.50-52 We were interested in examining the effect of Mig on ligand-induced calcium mobilization in eosinophils. As expected, eotaxin-1 and eotaxin-2 induced intracellular calcium changes in murine eosinophils and desensitized to each other (Figure 4A); however, Mig failed to both initiate calcium flux and inhibit eotaxin-2–induced calcium mobilization (Figure 4B). Calcium mobilization by PAF was also unaffected by pretreatment with Mig (Figure 4C). These results demonstrate that Mig blocks specific agonist-induced signal transduction events in eosinophils that are either independent of or downstream of calcium signaling.
CCR3 is required for Mig's inhibitory activity
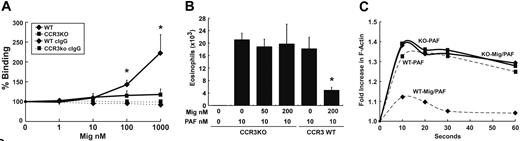
While a single publication has reported that a subset of human eosinophils expresses Mig's primary receptor CXCR3, we have not been able to detect CXCR3 on the surface of murine eosinophils.14,53 We were interested in further exploring this finding by testing the hypothesis that Mig's inhibitory action on eosinophils might be mediated by CCR3. In order to examine this, we generated hypereosinophilic mice that were deficient in CCR3 by mating IL-5 transgenic mice with CCR3 gene–targeted mice. We first aimed to determine if murine Mig directly bound to murine CCR3. Accordingly, we examined Mig's binding to wild-type and CCR3-deficient eosinophils by flow cytometry (Figure 5A). Notably, we detected dose-dependent binding of Mig to wild-type eosinophils. In contrast, Mig binding to CCR3-deficient eosinophils was not significantly different from the background staining seen with the isotype-matched control antibody (Figure 5A). These results are consistent with previous studies that have demonstrated that the human CXCR3 ligands (Mig, interferon-inducible protein 10 [IP-10], and interferon-inducible T-cell alpha chemoattractant [I-TAC]) bind to human CCR3.8,13
Having identified that Mig directly binds to CCR3, we next aimed to prove that CCR3 was required for Mig's inhibitory action. In order to examine this, we pretreated purified CCR3-deficient eosinophils with Mig and examined their chemotactic response to PAF. While Mig pretreatment markedly inhibited wild-type eosinophil responses toward PAF, Mig's inhibitory activity on PAF-induced chemotaxis was completely abrogated in CCR3 gene–targeted eosinophils (Figure 5B). Furthermore, the ability of Mig (200 nM) to inhibit PAF-induced F-actin formation was lost in CCR3-deficient eosinophils (Figure 5C). Collectively, these data establish that CCR3 is required for Mig's inhibitory activity on eosinophils.
Mig alters CCR3 ligand–receptor interaction
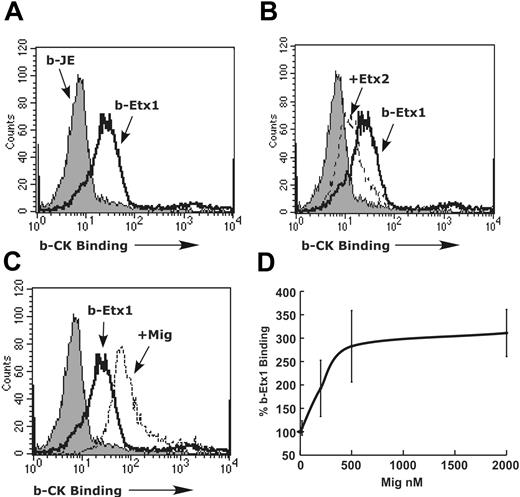
Previous studies have demonstrated that the human CXCR3 ligands (Mig, IP-10, and I-TAC) have a distinct binding site from the eotaxins on human CCR3.13 We thus wanted to investigate the effects of Mig on eotaxin binding to murine eosinophils. Accordingly, we measured binding of biotinylated eotaxin-1 to the surface of murine eosinophils by flow cytometry. We detected specific binding of eotaxin-1 (20 nM) to the surface of eosinophils when compared with binding of a control chemokine JE/CC chemokine ligand 2 (CCL2, 20 nM) (Figure 6A). Importantly, incubation with 10-fold excess unlabeled eotaxin-2 resulted in reduced binding of eotaxin-1 (Figure 6B). Remarkably, incubation with Mig (500 nM) resulted in markedly increased binding of eotaxin-1 to the surface of murine eosinophils (Figure 6C). Following incubation with Mig at 20, 200, 500, and 2000 nM, we detected dose-dependent and saturable increased binding of eotaxin-1 (Figure 6D). Together with the actin/Rac2 data, these results support a model where Mig induces a conformation change in CCR3 that initiates an inhibitory signal transduction cascade that results in reduced Rac activation, altered actin organization and assembly, and inhibition of agonist-induced chemotaxis.
Mig does not inhibit agonist-induced calcium mobilization. Calcium transients in purified murine eosinophils were measured by labeling cells with Fura-2 AM, and monitoring immediately following stimulation with 50 nM eotaxin-1, 25 nM eotaxin-2 (A-B), and 10 nM PAF (C). Prestimulation with Mig (200 nM) has no effect on eotaxin- (B) or PAF-induced calcium mobilization (C). Data are representative of 3 experiments.
Mig does not inhibit agonist-induced calcium mobilization. Calcium transients in purified murine eosinophils were measured by labeling cells with Fura-2 AM, and monitoring immediately following stimulation with 50 nM eotaxin-1, 25 nM eotaxin-2 (A-B), and 10 nM PAF (C). Prestimulation with Mig (200 nM) has no effect on eotaxin- (B) or PAF-induced calcium mobilization (C). Data are representative of 3 experiments.
Discussion
While most studies have focused on the positive effect of chemokines on cells, recent attention has been drawn to the negative (or inhibitory) effects of chemokines on various target cells. Understanding the inhibitory properties of chemokines has significant implications for disease therapy, yet there is a surprising paucity of studies concerning inhibitory mechanisms. In this study, we aimed to dissect the mechanism of Mig's potent eosinophil-specific inhibitory activity. Our results demonstrate several important and novel findings regarding the mechanism of a natural chemokine antagonist such as Mig. First, we demonstrate that Mig potently inhibits eosinophil transmigration toward both CCR3 and non-CCR3 ligands, demonstrating activity beyond receptor antagonism alone. Second, we demonstrate that Mig initiates an inhibitory signaling pathway that results in reduced agonist-induced Rac activation and impaired actin polymerization and localization. Importantly, Mig treatment alone results in abnormal actin organization, demonstrating a prominent role for Mig in the regulation of cytoskeletal rearrangement in murine eosinophils. It is interesting to note that Mig treatment results in impaired actin localization that resembles that seen in Rac2-deficient granulocytes.48 Consistent with this observation, we demonstrate that Rac2 is required for optimal CCR3-dependent chemoattraction and for Mig-induced inhibition. Furthermore, we demonstrate that Rac2 is not required for ligand-induced homologous desensitization of CCR3. Thus, CCR3 ligand–induced desensitization and Mig-induced inhibition are mediated by distinct mechanisms. Notably, the inhibitory activity of Mig occurs independently of calcium mobilization. This finding indicates that inhibition of calcium flux should not be used to screen for the presence of chemokine receptor inhibition since a powerful inhibitor such as Mig can induce profound effects independent of calcium signaling. Finally, using CCR3 gene–targeted cells, a role for CCR3 in mediating Mig's inhibitory activity is elucidated. Collectively, these data provide multiple lines of evidence that Mig generates negative signaling via CCR3. This is a surprising finding since CCR3 was previously regarded solely as a powerful activating receptor in eosinophils. Thus, depending upon which ligand engages CCR3, a completely different signaling cascade is engaged.
Mig requires CCR3 expression for inhibitory activity. (A) The dose-dependent binding of Mig to the surface of wild-type (♦) and CCR3-deficient (▪) cells is shown. Data represent mean ± SD percent mean channel fluorescence (chemokine binding) compared with no chemokine for 3 independent experiments combined. Staining with control antibody (dashed lines) is shown. *P < .05. (B) Mig does not inhibit CCR3-deficient eosinophil chemotaxis toward PAF. Cells were allowed to transmigrate following pretreatment with buffer or Mig. Data represent mean ± SD of eosinophils that migrated toward PAF (10 nM). *P < .05. The results are representative of 3 experiments. (C) Mig does not inhibit PAF-induced actin polymerization in CCR3-deficient eosinophils. Wild-type (dashed line) and CCR3-deficient (solid line) eosinophils were treated with PAF (10 nM, ▪), or Mig (200 nM) and PAF (♦) for the indicated period of time. Cells were fixed and stained with NBD-phallacidin. Relative F-actin content is expressed as the ratio of the mean channel fluorescence between eotaxin- and media alone–stimulated cells. A representative experiment is shown (n = 3).
Mig requires CCR3 expression for inhibitory activity. (A) The dose-dependent binding of Mig to the surface of wild-type (♦) and CCR3-deficient (▪) cells is shown. Data represent mean ± SD percent mean channel fluorescence (chemokine binding) compared with no chemokine for 3 independent experiments combined. Staining with control antibody (dashed lines) is shown. *P < .05. (B) Mig does not inhibit CCR3-deficient eosinophil chemotaxis toward PAF. Cells were allowed to transmigrate following pretreatment with buffer or Mig. Data represent mean ± SD of eosinophils that migrated toward PAF (10 nM). *P < .05. The results are representative of 3 experiments. (C) Mig does not inhibit PAF-induced actin polymerization in CCR3-deficient eosinophils. Wild-type (dashed line) and CCR3-deficient (solid line) eosinophils were treated with PAF (10 nM, ▪), or Mig (200 nM) and PAF (♦) for the indicated period of time. Cells were fixed and stained with NBD-phallacidin. Relative F-actin content is expressed as the ratio of the mean channel fluorescence between eotaxin- and media alone–stimulated cells. A representative experiment is shown (n = 3).
Mig alters CCR3 receptor-ligand interaction. (A) Binding of 10 nM biotinylated eotaxin-1 (b-Etx1, black line) without (A) or with 200 nM eotaxin-2 (B) to the surface of eosinophils compared with control chemokine (biotinylated JE, b-JE, filled histogram) is shown. Representative histogram from 3 experiments is shown. (C) Binding of eotaxin-1 (black line) with or without 500 nM Mig (dashed line) is shown. A representative histogram is shown (n = 3). (D) Percent eotaxin-1 binding in the presence of 20 to 2000 nM Mig compared with eotaxin-1 alone is shown. Data represent mean ± SD of mean channel fluorescence of eotaxin-1 binding in the presence of Mig compared with eotaxin-1 alone (n = 3 experiments).
Mig alters CCR3 receptor-ligand interaction. (A) Binding of 10 nM biotinylated eotaxin-1 (b-Etx1, black line) without (A) or with 200 nM eotaxin-2 (B) to the surface of eosinophils compared with control chemokine (biotinylated JE, b-JE, filled histogram) is shown. Representative histogram from 3 experiments is shown. (C) Binding of eotaxin-1 (black line) with or without 500 nM Mig (dashed line) is shown. A representative histogram is shown (n = 3). (D) Percent eotaxin-1 binding in the presence of 20 to 2000 nM Mig compared with eotaxin-1 alone is shown. Data represent mean ± SD of mean channel fluorescence of eotaxin-1 binding in the presence of Mig compared with eotaxin-1 alone (n = 3 experiments).
Recent studies have identified mechanisms by which different ligands can induce distinct conformations of CCR2 and CCR7.15,17 In these studies, the ligands were either agonists or partial agonists, resulting in differential receptor desensitization states. In our study, we investigate the mechanism by which an activating and inhibitory chemokine results in opposing activity on eosinophils using the same receptor. We propose a model in which binding of Mig to CCR3 promotes alterations in the receptor that are associated with initiation of negative signaling. In particular, Mig inhibits agonist-induced Rac activation and disorganization of the F-actin cytoskeleton, which promote cellular inhibition to multiple stimuli. In contrast, engagement of CCR3 with activating ligands (eotaxins) induces a marked calcium flux, activation of Rac, and F-actin reorganization and polymerization. It is likely that Mig induces a conformational change in CCR3 that induces the inhibitory signaling. This is supported by our finding that Mig increases eotaxin-1 binding to the surface of murine eosinophils without changing the surface expression of CCR3,14 presumably by inducing a receptor conformation that alters agonist-receptor interaction. Mig and eotaxin-1 may directly interact, resulting in altered receptor-ligand interaction, but this would not explain Mig's inhibitory activity on non-CCR3–mediated chemotaxis. Competitive binding studies using human CXCR3 ligands and human CCR3-expressing cells have yielded conflicting results.8,13 While the CXCR3 ligand I-TAC has been shown to effectively displace labeled eotaxin-1, the concentration of Mig required to compete off a similar percentage of labeled eotaxin-1 was 100-fold greater.8 A more recent study demonstrated that I-TAC could not effectively compete with labeled eotaxin-1 for binding to CCR3.13 These divergent results may be related to the different transfected cell lines used. Our studies, using murine Mig and primary murine eosinophils, are consistent with distinct binding sites for Mig and the eotaxins on CCR3.13
Consistent with previous studies in neutrophils, Rac2 deficiency in eosinophils resulted in defects in directed cell movement, a function requiring actin cytoskeletal rearrangements. Our data demonstrate an important regulatory role for Rac2 in actin remodeling in eosinophils. We also demonstrate that Rac2 is required not only for optimal actin polymerization and chemotaxis in eosinophils in response to an agonist, but also for Mig's inhibitory activity, supporting the concept that Rac2 is a key regulator of eosinophil chemoattraction. The inhibitory signaling induced by Mig may be promoted by the interaction of GTPases with guanine-nucleotide exchange factors (GEFs) that catalyze the GDP-GTP exchange reaction. GEFs contain pleckstrin homology domains and are regulated by the products and substrates of phosphatidylinositol 3–kinase (PI3K).54,55 Mig may control Rac activation by regulating GEF or PI3K activity.
These results support a new paradigm concerning eosinophil chemokine pathways by demonstrating that eosinophil chemokines can induce diverse signaling pathways. Notably, we propose that the major eosinophil chemokine receptor CCR3, previously identified solely as a powerful activating receptor in eosinophils, can transmit positive or negative signals depending upon the ligand engaged. These results call attention to the importance of dissecting the mechanism of inhibitory chemokine activity.
Prepublished online as Blood First Edition Paper, March 31, 2005; DOI 10.1182/blood-2005-02-0489.
Supported in part by National Institutes of Health (NIH) R01 AI42242, R01 AI45898, and P01 HL076383, and the Burroughs Wellcome Fund.
P.F. and M.R. designed the research; P.F., H.Z., and N.Z. performed the research; D.W. provided reagents; P.F., H.Z., N.Z., D.W., and M.R. analyzed data; P.F. and M.R. wrote the manuscript; and N.Z. and D.W. reviewed the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Dr Marie-Dominique Filippi for helpful discussions; Laura Koch and Amy Hajek for technical assistance; and Andrea Lippelman for assistance with the preparation of this manuscript.
The authors declare that they have no competing financial interests.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal