Abstract
Triggering of the T-cell receptor (TCR) can produce very different responses, depending on the nature of the major histocompatibility complex/antigen peptide (MHCp) ligand. The molecular mechanisms that permit such fine discrimination are still unknown. We show here that an epitope in the cytoplasmic tail of the TCR CD3ϵ subunit, recognized by antibody APA1/1, is only detected when the TCR is fully activated. Exposure of the APA1/1 epitope is shown to be fast and independent of tyrosine kinase activity and that it takes place even when T cells are stimulated at 0°C. These results suggest that APA1/1 detects a conformational change in the TCR. APA1/1 staining concentrates in a restricted area of the immunologic synapse. Most important, we show that full agonist, but not partial agonist, peptides induce exposure of the APA1/1 epitope, indicating a correlation between the induction of the conformational change in the TCR and full T-cell activation. Finally, the conformational change is shown to occur in T cells that are being stimulated by antigen in vivo. Therefore, these results demonstrate that the TCR undergoes a conformational change on MHCp binding in vitro and in vivo, and they establish a molecular correlate for productive TCR engagement. (Blood. 2005;106:601-608)
Introduction
When T cells recognize antigen-presenting cells (APCs) that bear an appropriate antigen, large-scale rearrangement of the T-cell cytoskeleton occurs, coupled to a redistribution of plasma membrane and cytoplasmic molecules. This reorganization results in the formation of what is known as an immunologic synapse (IS).1,2 Antibody-staining experiments and time-lapse confocal microscopy have demonstrated that central and peripheral supramolecular activation clusters (cSMACs and pSMACs) form at the IS.3 Although the cSMAC contains the T-cell receptor (TCR) and signaling molecules such as protein kinase C θ (PKCθ), the pSMAC is formed by a ring of integrins, such as leukocyte factor antigen 1 (LFA1) (CD11a/CD18), and accompanying cytoskeletal proteins, such as talin.3 It has been proposed that the formation of the IS serves to sustain TCR signaling, which is necessary for full T-cell activation.1,2 However, some studies indicate that TCR signaling takes place before the IS is formed,4 whereas others indicate that the IS is a site for TCR internalization and down-modulation of the activation signals.5
Much of the evidence supporting the need to form the IS for T-cell activation derives from the observation that antagonist and partial agonist peptides do not form bona fide ISs.2,6,7 Furthermore, TCR engagement with a partial agonist peptide results in a pattern of intracellular signaling distinct from that induced with a full-agonist peptide. Thus, stimulation with a partial agonist induces only partial phosphorylation of the CD3ζ and CD3ϵ chains and the recruitment, but not the phosphorylation, of ZAP70 (zeta-associated protein of 70 kDa),8,9 resulting in the activation of a subset of the downstream signaling pathways that are activated by an agonist peptide.10 Antagonist peptides bind to the TCR with lower affinities and higher dissociation rates than full agonists,11 consistent with a kinetic model of TCR triggering in which more transient occupancy of the TCR leads to partial signaling. However, in addition to altered kinetic parameters, occupancy of the TCR by antagonist peptides may produce a different structural arrangement than occupancy by agonist peptides.
The existence of conformational changes in the TCR was first proposed by Janeway and colleagues12 based on the TCR-induced cocapping of CD4.12 Although structural data generally preclude the existence of large rearrangements in the ectodomains of the TCRα/β heterodimer,13,14 we recently demonstrated that ligand binding to the TCR induces a conformational change in the cytoplasmic tail of CD3ϵ that exposes a proline-rich sequence.15 The exposed sequence can consequently interact with Nck. Blockade of the proline-rich sequence of CD3ϵ in vitro results in inefficient T-cell activation, suggesting that the conformational change in the TCR CD3ϵ subunit is important for the activation of downstream intracellular signaling pathways.15
We now show that antibody or major histocompatibility complex/antigen peptide (MHCp) engagement of the TCR promotes the exposure of an epitope recognized by a monoclonal antibody (mAb) (APA1/1) that binds to the cytoplasmic tail of CD3ϵ. Furthermore, we show that APA1/1 stains T cells in the lymph nodes of mice exposed to antigen, suggesting that the antibody can distinguish T cells that are being stimulated by MHCp in vivo. More important, unlike conventional anti-TCR antibodies, APA1/1 discriminates between the ISs formed between T cells and APCs primed with full-agonist peptides and the ISs formed by APCs primed with partial agonists. Thus, we conclude that the conformational change in the TCR may underlie its distinction between full agonists and partial agonists.
Materials and methods
Cells and mice
The human Jurkat T-cell line and the lymphoblastoid B-cell line Raji were cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS; Sigma, St Louis, MO). CH7C17, a Jurkat cell transfectant expressing the TCRα and TCRβ chains from the DR1-HA-responsive human T cell clone HA1.7, and DAP-DR1-ICAM, murine DAP fibroblasts transfected with human intercellular adhesion molecule-1 (ICAM-1) and HLA-DR1, were generously provided by Dr M. Owen (Cancer Research UK, London, United Kingdom). C57BL/6, OT-I (OT-I TCR transgenic on a C57BL/6 background),16 and OT-I RAG2-/- mice were bred and maintained free of pathogens at our animal facility.
Antibodies and other reagents
Anti-CD3ϵ mouse mAb APA1/1 has been described previously,17 as has rabbit anti-CD3ζ antiserum 448.18 Biotin anti-mouse TCRβ H57-597, mouse anti-human CD3 Leu4, anti-mouse CD4 phycoerythrin (PE), CD4 fluorescein isothiocyanate (FITC), CD8 PE, and biotin CD8 were purchased from BD PharMingen (San Diego, CA). Hamster anti-mouse CD3 145-2C11 hybridoma was from the American Type Culture Collection (ATCC; Manassas, VA), anti-human PKCθ was from Transduction Laboratories (Lexington, KY), anti-phosphotyrosine 4G10 was from Upstate Biotechnology (Lake Placid, NY), anti-mouse α-tubulin (DM1A) was from Sigma, and goat anti-CD3ϵ M20 (recognizing the C-terminal end) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Hamster anti-murine CD11c mAb N418 was a gift of C. Ardavín (Centro Nacional de Biotecnología, Madrid, Spain), and the anti-H-2Kb-ovalbumin (OVA) mAb has been described previously.19 Staphylococcus aureus enterotoxin E (SEE) was from Toxin Technologies (Sarasota, FL), the inhibitor of the Src family kinases (PP2) was from Calbiochem (San Diego, CA), and the dimer H-2Kb/immunoglobulin (Ig) was from BD PharMingen. OT-I TCR agonist (OVAp, SIINFEKL) and antagonist (E1, EIINFEKL) and HA1.7 TCR agonist (HA307-319, PKYVKQNTLKLAT) and antagonist (PKYVKQNTLALAT) peptides were synthesized at our facility by the f-moc method.
T-cell stimulation and flow cytometry
Spleen cells from 6-week-old OT-I RAG2-/- mice were stimulated with H-2Kb/Ig dimers loaded with the indicated peptide for 1 minute at 37°C. Anti-mouse immunoglobulin-PE (PharMingen) was added for 15 minutes at 37°C, and the cells were fixed and permeabilized using the Cytofix/Cytoperm kit (PharMingen) according to the manufacturer's instructions. Biotin-labeled APA1/1 was added at a concentration of 4 μg/mL for 30 minutes on ice. Cells were stained with specific fluorochrome-conjugated antibodies and analyzed by flow cytometry in a FACSCalibur flow cytometer using the CellQuest software (Becton Dickinson, San Jose, CA). For antibody stimulation, spleen or lymph node cells were incubated with 10 μg/mL 145-2C11 for 10 minutes at 37°C and were directly stained on ice with PE-labeled anti-CD4 or anti-CD8 antibodies. APA1/1 staining was analyzed after fixation and permeabilization, as for H-2Kb/Ig dimer stimulation.
Pull-down assay
For the glutathione-S-transferase (GST)-Nck pull-down assay, cell lysates were first precleared with GST adsorbed to glutathione-Sepharose (Amersham Biosciences, Freiburg, Germany) before affinity chromatography with the amino-terminal SH3 domain of Nckα fused to GST adsorbed to glutathione-Sepharose.15
Cell conjugation and immunofluorescence labeling
Jurkat/Raji conjugates. To distinguish APCs from T cells, Raji cells were loaded with 10 μM chloromethyl aminocoumarin (CMAC) for 30 minutes at 37°C, washed, and resuspended in RPMI 1640 with 10% FBS. Cells were then incubated for 15 minutes in the presence of 1 μg/mL SEE. Jurkat cells were mixed with an equal number of Raji cells in a final volume of 50 μL and were incubated at 37°C for 15 minutes. Conjugates were plated on poly-L-lysine-coated slides, fixed for 10 minutes in 2% formaldehyde-Hanks balanced salt solution (HBSS) and were stained with the appropriate antibodies, using goat anti-mouse or rabbit Alexa488, Alexa594, or Alexa647 (Caltag, Burlingame, CA) as the secondary antibodies. Cells were visualized with an Axiovert 200 microscope (Carl Zeiss, Oberkochen, Germany) or with a Zeiss Radiance 2000 confocal microscope and were analyzed with the ImageJ 1.33v software (National Institutes of Health, Bethesda, MD). Images were acquired with a charge-coupled device (CCD) camera SPOT RT Slider (Diagnostic Instruments, Sterling Heights, MI).
CH7C17/DAP-DR1-ICAM conjugates. The APCs DAP-DR1-ICAM were cultured on coverslips with 10 μM HA307-319 or HAK316A peptides for 24 hours at 37°C. Afterward, they were incubated with CH7C17 at a 1:1 ratio for 15 minutes at 37°C. Cells were fixed and stained as described in the previous paragraph for Jurkat/Raji conjugates.
Spleen cell conjugates. Unfractionated spleen cells from OT-I C57BL/6 mice were incubated with 2 μM of the indicated peptide for 2 hours at 37°C. The cells were plated on poly-L-lysine-coated slides. Antibody staining was performed as described earlier in this section for Jurkat/Raji conjugates.
Confocal microscopy
T-cell/APC conjugates were identified based on cell morphology under differential interference contrast (DIC) microscopy with blue fluorescence for CMAC-labeled APCs (Jurkat/Raji conjugates) and green fluorescence for antibody marker-labeled APCs (spleen cell conjugates). The proportion of conjugates with APA1/1 or CD3 redistributed to the T-cell/APC contacts was calculated by randomly choosing 100 different conjugates. Confocal microscopy was carried out on a Zeiss Radiance 2000 using a 63 × 1.4 oil Plan-Apochomat objective lens. Image acquisition was with the Lasersharp 2000 5.2 software (Zeiss) and analysis with the Image J 1.33v software.
Immunohistochemical studies
Lymph nodes were fixed with 4% paraformaldehyde solution in phosphate-buffered saline (PBS), washed with PBS, and incubated overnight in a 30% sucrose solution in PBS at 4°C. Lymph nodes were embedded in OCT compound (Tissue-Tec; Miles Scientific, Naperville, IL), and 10-μm cryostat sections were obtained using a 2800 Frigocut N cryostat (Leica, Nussloch, Germany). Sections were fixed again with 4% paraformaldehyde in PBS, quenched with 20 mM NH4Cl, and stained for 1 hour with antibodies in a solution containing 1% FBS. Secondary antibodies were prepared as described in “Cell conjugation and immunofluorescence labeling,” and the sections were mounted in fluorescent mounting medium (DAKO, Carpinteria, CA).
Results
Conformational changes in the TCR revealed by exposure of the APA1/1 epitope
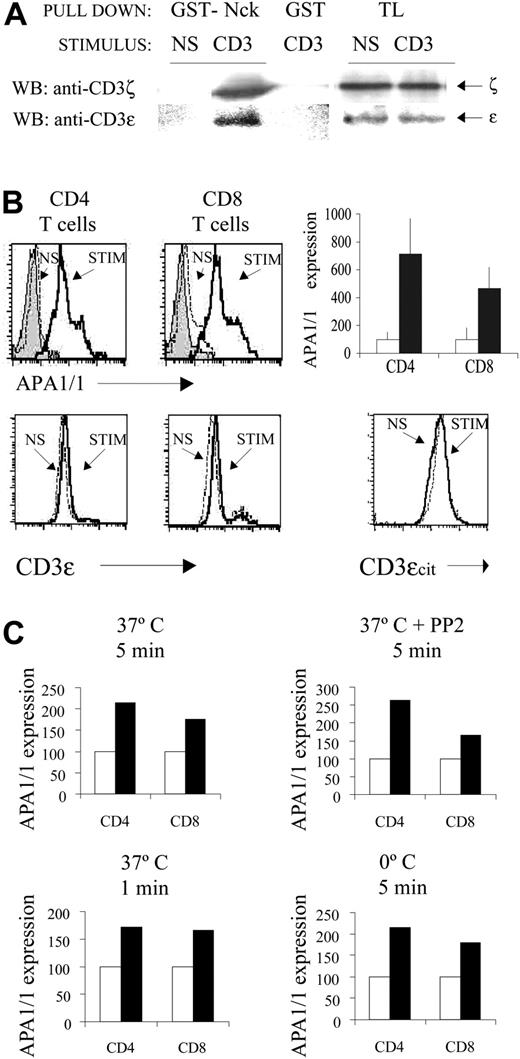
We have previously revealed the conformational change in the intracellular domain of the TCR using a pull-down assay with GST-Nck fusion protein.15 Consistent with these earlier results, stimulation of spleen T cells with anti-CD3 antibody induced the binding of Nck to the TCR (Figure 1A). Because the mAb APA1/1 binds in the proximity of the proline-rich sequence of CD3ϵ20 and APA1/1 inhibits the Nck-CD3ϵ interaction,15 we investigated whether the epitope recognized by APA1/1 was itself exposed on TCR ligand binding. Stimulation of spleen CD4+ T cells and CD8+ T cells with anti-CD3 antibody clearly increased intracellular immunostaining by APA1/1 in comparison with nonstimulated cells or antibody controls, as detected by flow cytometric analysis (Figure 1B). A shoulder in both the CD4+ and the CD8+ T cell populations was consistently observed, suggesting heterogeneity in the response to anti-CD3 antibody. The increase in APA1/1 immunofluorescence was not caused by the increased expression of the TCR complex or CD3ϵ protein because less TCR was apparent at the cell surface (data not shown) and the total CD3ϵ expression in permeabilized cells remained unchanged (Figure 1B). Furthermore, accessibility of a different cytoplasmic epitope, contained within the C-terminal 15 amino acids of CD3ϵ, did not change on TCR triggering (Figure 1B). These flow cytometric analyses were confirmed by immunoblotting, which demonstrated that total CD3ϵ remained unchanged after brief stimulation with anti-CD3 antibody (Figure 1A). These results, therefore, indicate that TCR engagement induces exposure of the APA1/1 epitope in the absence of a concomitant change in TCR protein levels.
Antibody engagement of the TCR induces the exposure of the APA1/1 epitope in primary cells. (A) Pull-down assay. Spleen cells from C57BL/6 mice stimulated for 5 minutes with 10 μg/mL soluble anti-CD3 145-2C11 or left nonstimulated (NS) were lysed in Brij96, and the lysate was incubated with GST-Nck beads or with GST. Precipitated proteins were subjected to immunoblotting and were probed first with anti-CD3ζ and subsequently with anti-CD3ϵ M20. Total lysate (TL) was run as a control of loading. (B) Induced exposure of the APA1/1 epitope. Spleen cells from C57BL/6 mice were stimulated (STIM) with soluble anti-CD3 for 5 minutes at 37°C or were left nonstimulated (NS), fixed, permeabilized, and stained with APA1/1. Staining with an irrelevant IgG1 antibody was carried out in parallel on stimulated cells as a control (gray shaded curves). APA1/1 immunofluorescence on gated CD4+ and CD8+ T cells is shown. The diagram to the right shows the quantitative increase in mean fluorescence intensity (MFI) after stimulation (in reference to the nonstimulated cells, □) and SD from data collected in triplicate. To quantify total TCR levels, permeabilized spleen T cells were stained either with anti-CD3ϵ antibody 145-2C11 or with antibody M20, recognizing the C-terminal end of CD3ϵ (CD3ϵcit). (C) Protein tyrosine kinase (PTK) and temperature independence of APA1/1 epitope exposure. Spleen T cells were stimulated with anti-CD3 for 5 minutes at 37°C in the presence or absence of 20 μM PP2, for 1 minute at 37°C, or for 5 minutes on ice. For PP2 treatment, cells were preincubated with the drug for 30 minutes before stimulation. ▪ indicates stimulated samples; □, nonstimulated samples.
Antibody engagement of the TCR induces the exposure of the APA1/1 epitope in primary cells. (A) Pull-down assay. Spleen cells from C57BL/6 mice stimulated for 5 minutes with 10 μg/mL soluble anti-CD3 145-2C11 or left nonstimulated (NS) were lysed in Brij96, and the lysate was incubated with GST-Nck beads or with GST. Precipitated proteins were subjected to immunoblotting and were probed first with anti-CD3ζ and subsequently with anti-CD3ϵ M20. Total lysate (TL) was run as a control of loading. (B) Induced exposure of the APA1/1 epitope. Spleen cells from C57BL/6 mice were stimulated (STIM) with soluble anti-CD3 for 5 minutes at 37°C or were left nonstimulated (NS), fixed, permeabilized, and stained with APA1/1. Staining with an irrelevant IgG1 antibody was carried out in parallel on stimulated cells as a control (gray shaded curves). APA1/1 immunofluorescence on gated CD4+ and CD8+ T cells is shown. The diagram to the right shows the quantitative increase in mean fluorescence intensity (MFI) after stimulation (in reference to the nonstimulated cells, □) and SD from data collected in triplicate. To quantify total TCR levels, permeabilized spleen T cells were stained either with anti-CD3ϵ antibody 145-2C11 or with antibody M20, recognizing the C-terminal end of CD3ϵ (CD3ϵcit). (C) Protein tyrosine kinase (PTK) and temperature independence of APA1/1 epitope exposure. Spleen T cells were stimulated with anti-CD3 for 5 minutes at 37°C in the presence or absence of 20 μM PP2, for 1 minute at 37°C, or for 5 minutes on ice. For PP2 treatment, cells were preincubated with the drug for 30 minutes before stimulation. ▪ indicates stimulated samples; □, nonstimulated samples.
Exposure of the APA1/1 epitope was not affected by inhibiting TCR-induced tyrosine kinase activity with the src-kinase inhibitor PP2 at a concentration that completely abrogated tyrosine phosphorylation (Figure 1C; data not shown). Moreover, the APA1/1 epitope was exposed very early after TCR engagement (1 minute) and also when the cells were stimulated on ice (Figure 1C). These results suggest that exposure of the APA1/1 epitope is independent of the activation of intracellular signaling pathways and independent of the cell's metabolic activity, corroborating the observations previously made in the GST-Nck pull-down assay15 and indicating that the APA1/1 antibody can be used to trace the conformational change in the TCR.
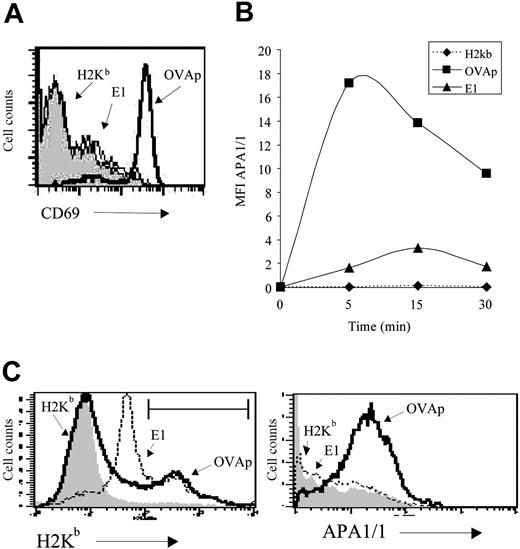
Stimulation of CD8+T cells with agonist MHCp dimer but not with partial agonist MHCp dimer exposes the APA1/1 epitope. (A) Induction of CD69 expression by agonist and partial agonist H-2Kb/Ig dimers. Spleen cells from OT-IxRAG2-/- mice were stimulated with 500 ng/mL of H-2Kb/Ig dimer unloaded (H-2Kb; gray shaded curve) or loaded with the agonist OVAp peptide (SIINFEKL) or with the partial agonist peptide E1 (EIINFEKL). Expression of CD69 in gated CD8+ T cells was analyzed 24 hours later. (B) Time course of APA1/1 epitope exposure. Spleen cells from OT-I mice were stimulated at room temperature for the times indicated with 500 ng/mL of the H-2Kb/Ig dimer loaded with the indicated peptides. APA1/1 immunofluorescence (expressed as MFI) was recorded on the H-2Kb/Ig dimer+ cells. (C) Binding of OVAp- and E1-loaded H-2Kb/Ig dimer to OT-I CD8+ T cells. Spleen cells from OT-I mice were incubated for 5 minutes at room temperature with 500 ng/mL of the H-2Kb/Ig dimers loaded with the indicated peptides and triple stained for Thy1, the H-2Kb/Ig dimer, and APA1/1. The Thy1+ population was analyzed for peptide H-2Kb/Ig binding (left). The brightest population for OVAp-H-2Kb/Ig binding (22%) and E1-H-2Kb/Ig binding (28%) was gated as indicated and reanalyzed for APA1/1 expression (right).
Stimulation of CD8+T cells with agonist MHCp dimer but not with partial agonist MHCp dimer exposes the APA1/1 epitope. (A) Induction of CD69 expression by agonist and partial agonist H-2Kb/Ig dimers. Spleen cells from OT-IxRAG2-/- mice were stimulated with 500 ng/mL of H-2Kb/Ig dimer unloaded (H-2Kb; gray shaded curve) or loaded with the agonist OVAp peptide (SIINFEKL) or with the partial agonist peptide E1 (EIINFEKL). Expression of CD69 in gated CD8+ T cells was analyzed 24 hours later. (B) Time course of APA1/1 epitope exposure. Spleen cells from OT-I mice were stimulated at room temperature for the times indicated with 500 ng/mL of the H-2Kb/Ig dimer loaded with the indicated peptides. APA1/1 immunofluorescence (expressed as MFI) was recorded on the H-2Kb/Ig dimer+ cells. (C) Binding of OVAp- and E1-loaded H-2Kb/Ig dimer to OT-I CD8+ T cells. Spleen cells from OT-I mice were incubated for 5 minutes at room temperature with 500 ng/mL of the H-2Kb/Ig dimers loaded with the indicated peptides and triple stained for Thy1, the H-2Kb/Ig dimer, and APA1/1. The Thy1+ population was analyzed for peptide H-2Kb/Ig binding (left). The brightest population for OVAp-H-2Kb/Ig binding (22%) and E1-H-2Kb/Ig binding (28%) was gated as indicated and reanalyzed for APA1/1 expression (right).
Conformational change of TCR induced by full, but not partial, agonist ligands
It was important to confirm that the conformational change in the TCR could be detected not only after stimulation with anti-CD3 antibodies but also with MHCp. To investigate this, we used spleen T cells from transgenic mice with the H-2Kb-restricted OT-I TCR. Stimulation of OT-I cells with H-2Kb/Ig dimers loaded with the OVA agonist peptide (OVAp, SIINFEKL) induced expression of the CD69 activation marker (Figure 2A). In contrast, stimulation of the cells with unloaded H-2Kb/Ig dimers or with dimers loaded with the partial agonist/antagonist E1 peptide (EIINFEDKL)16,21,22 failed to activate CD69 expression. Using the same set of peptide-loaded H-2Kb/Ig dimers, we found that the H-2Kb/Ig dimer loaded with the agonist OVAp, but not the empty dimer or the dimer loaded with E1, induced a shift in the mean intensity of APA1/1 fluorescence within 5 minutes (Figure 2B). The experiment was performed at room temperature to reduce down-modulation of the TCR promoted by the H-2Kb:Ig-OVAp agonist ligand. Although BIAcore experiments have shown that H-2Kb-E1 has a lower affinity for the OT-I TCR than H-2Kb-OVAp,11 the failure to detect the APA1/1 epitope after E1 engagement was not the result of poor binding. Indeed, a well-defined population of T cells bound to the E1-loaded H-2Kb/Ig dimer was detected (Figure 2C, left). However, cells that bound to H-2Kb-OVAp presented more intense APA1/1 immunofluorescence than cells bound to H-2Kb-E1 (Figure 2C, right). These results indicate that the conformational change revealed by exposure of the APA1/1 epitope in CD3ϵ is induced after productive engagement of the TCR by its physiological ligand.
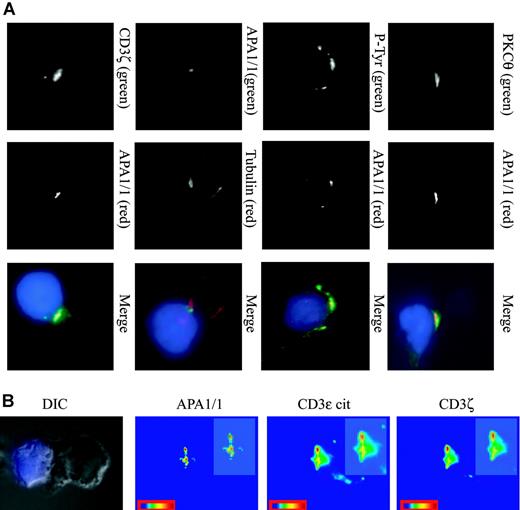
Restricted area of the cSMAC defined by the APA1/1 epitope. (A) Localization of APA1/1 staining in the cSMAC. Jurkat T cells were incubated for 15 minutes with SEE-treated Raji APCs. The APCs were prestained with CMAC (blue), and the T-cell/APC conjugates were stained with the markers indicated in green (top row) and red (middle row). Cell conjugates were examined with a charge-coupled device (CCD) camera at a magnification of 100 ×. P-Tyr indicates antiphosphotyrosine antibody 4G10. (B) A differential interference contrast (DIC) image of the T cell/APC conjugate is shown to illustrate the position of the IS. APA1/1 marks a restricted area within the cSMAC. Jurkat-Raji cell conjugates were prepared as above and triple stained for APA1/1 (green channel), CD3ϵ cytoplasmic tail M20 (red channel), and CD3ζ (far red channel). Each channel was analyzed separately to provide a color-coded scale of intensities (red represents the strongest signal). (insets) 1.5-fold magnifications of the IS. Images were acquired with a Zeiss Axiovert 200 microscope.
Restricted area of the cSMAC defined by the APA1/1 epitope. (A) Localization of APA1/1 staining in the cSMAC. Jurkat T cells were incubated for 15 minutes with SEE-treated Raji APCs. The APCs were prestained with CMAC (blue), and the T-cell/APC conjugates were stained with the markers indicated in green (top row) and red (middle row). Cell conjugates were examined with a charge-coupled device (CCD) camera at a magnification of 100 ×. P-Tyr indicates antiphosphotyrosine antibody 4G10. (B) A differential interference contrast (DIC) image of the T cell/APC conjugate is shown to illustrate the position of the IS. APA1/1 marks a restricted area within the cSMAC. Jurkat-Raji cell conjugates were prepared as above and triple stained for APA1/1 (green channel), CD3ϵ cytoplasmic tail M20 (red channel), and CD3ζ (far red channel). Each channel was analyzed separately to provide a color-coded scale of intensities (red represents the strongest signal). (insets) 1.5-fold magnifications of the IS. Images were acquired with a Zeiss Axiovert 200 microscope.
APA1/1 epitope expressed by a subset of TCRs located in the IS
To study the cellular distribution of TCR molecules that expose the APA1/1 epitope during antigen presentation, we chose Jurkat T cells and Raji lymphoblastoid B cells as APCs. In the presence of an appropriate superantigen (SEE), these cells have been shown to form a well-defined, mature IS.23 Interestingly, the APA1/1 epitope was clearly associated with the IS and colocalized with PKCθ and tyrosine-phosphorylated proteins (Figure 3A). Moreover, the T-cell microtubule organizing center (MTOC) was found proximal to the APA1/1-stained area (α-tubulin staining). However, it appeared that the distribution of the APA1/1 epitope was more restricted in the IS than the PKCθ and tyrosine-phosphorylated proteins. This effect was also observed when the T-cell/APC synapse was costained with APA1/1 and another TCR marker, CD3ζ (Figure 3A). Thus, it seemed that the APA1/1 antibody defined a central area within the cSMAC. Finally, when APA1/1 immunofluorescence in the IS was quantitated, it was again clear that the pattern of exposure of the APA1/1 epitope was restricted in comparison with the areas of the cSMAC stained with anti-CD3ζ or with an antibody against the C-terminal end of CD3ϵ (Figure 3B). These results suggest that the restricted pattern of APA1/1 staining in the IS was not caused by weak binding but rather that only a fraction of the TCR present in the IS undergoes the conformational change that results in exposure of the APA1/1 epitope.
Distinction of productive ISs by induced expression of APA1/1 epitope
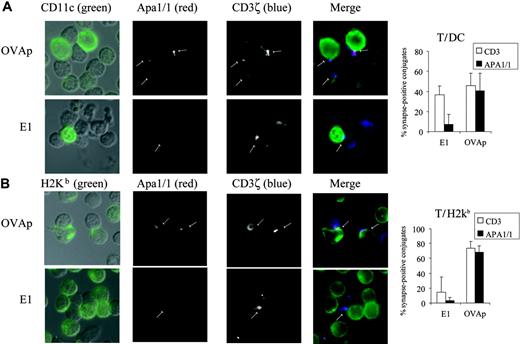
Exposure of the APA1/1 epitope occurred on binding to the TCR of H-2Kb dimers complexed to an agonist peptide (OVAp) but not to a partial agonist (E1; Figure 2), Hence, we sought to determine whether both types of ligand induced a similar type of synapse in primary T cells. To this end, total spleen cells from OT-I TCR transgenic mice were cultured ex vivo with both peptides, and the formation of synapses between OT-I T cells and APCs was analyzed. The OT-I T cells formed conjugates with dendritic cells (DCs) identified with the CD11c marker (Figure 4A). In almost half the T-cell/DC conjugates, the TCR was localized in the IS (visualized with anti-CD3ζ), and it was difficult to distinguish between the 2 peptide ligands (35% of synapses were labeled when stimulated with E1 and 45% when stimulated with OVAp; Figure 4A). However, exposure of the APA1/1 epitope was clearly dependent on the type of stimulus. Thus, APA1/1 was only detected in 8% of the ISs from T-cell/DC conjugates formed on stimulation with E1 compared with up to 40% on stimulation with OVAp. In synapses labeled for CD3ζ and APA1/1, APA1/1 staining was more restricted, again indicating that only a fraction of the TCR population undergoes this conformational change at one time (Figure 4A). To verify that at these synapses OT-I T cells were indeed recognizing APCs displaying the OVA peptides, we stained the cells with the 25-D1.16 antibody that specifically recognizes H-2Kb complexed to OVA. More than 95% of the APA1/1-labeled ISs were established with cells recognized by 25-D1.16 (data not shown). The 25-D1.16 antibody also labeled APCs loaded with both peptides (OVA and E1), and the effect of the quality of the antigen peptide on the formation of ISs containing APA1/1 and TCR corroborated the observations made with the DC marker (Figure 4B). Thus, to a greater degree than the general TCR marker anti-CD3ζ, APA1/1 made a clear distinction between agonist- and partial agonist-induced synapses. This distinction was especially important when DCs represented the APCs, a phenomenon that could be related to the fact that DCs can form functional synapses with T cells even in the absence of antigen and MHC.24
APA1/1 epitope discerns synapses of CD8+T cells stimulated ex vivo with agonist but not with partial agonist peptides. (A, left) T-cell/DC synapses. Total spleen OT-I cells were incubated for 2 hours with 2 μM of the peptides indicated. T-cell/APC conjugates were identified in DIC images (left column) superimposed with the green marker. T-cell/DC synapses were visualized by antibody staining for CD11c (DC marker, green) and CD3ζ (T cell marker, blue). Exposure of the APA1/1 epitope is shown in red and indicated with arrows. (Right) Quantitation of T-cell/DC synapses. The percentage of T-cell/DC conjugates forming synapses positive for CD3ζ (□) and for APA1/1 (▪) is shown as the mean ± SD of 3 independent fields with a total of more than 30 cells for each data point. (B) Total T-cell/APC synapses. Samples of the experiment shown in panel A were labeled with the 25.D1.16 antibody, specific for OVA peptide-loaded H-2Kb, to detect all APCs bearing the OT-I TCR ligands. Quantitation of total T-cell/APC synapses was performed as in panel A. Images were acquired with a Zeiss Axiovert 200 microscope with a CCD camera.
APA1/1 epitope discerns synapses of CD8+T cells stimulated ex vivo with agonist but not with partial agonist peptides. (A, left) T-cell/DC synapses. Total spleen OT-I cells were incubated for 2 hours with 2 μM of the peptides indicated. T-cell/APC conjugates were identified in DIC images (left column) superimposed with the green marker. T-cell/DC synapses were visualized by antibody staining for CD11c (DC marker, green) and CD3ζ (T cell marker, blue). Exposure of the APA1/1 epitope is shown in red and indicated with arrows. (Right) Quantitation of T-cell/DC synapses. The percentage of T-cell/DC conjugates forming synapses positive for CD3ζ (□) and for APA1/1 (▪) is shown as the mean ± SD of 3 independent fields with a total of more than 30 cells for each data point. (B) Total T-cell/APC synapses. Samples of the experiment shown in panel A were labeled with the 25.D1.16 antibody, specific for OVA peptide-loaded H-2Kb, to detect all APCs bearing the OT-I TCR ligands. Quantitation of total T-cell/APC synapses was performed as in panel A. Images were acquired with a Zeiss Axiovert 200 microscope with a CCD camera.
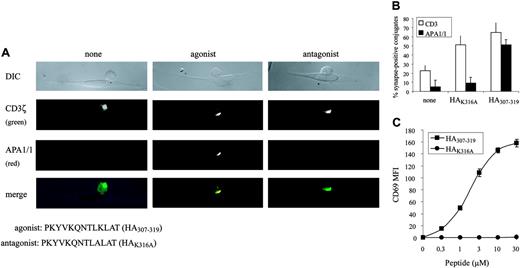
Given these results, we have found a correlation between the formation of ISs containing APA1/1—indicative of a TCR conformational change—and the activation of T cells. To demonstrate a correlation between the formation of a productive IS and the conformational change in the TCR in a different T-cell/APC system, thus adding general validity to our findings, an MHC class 2-restricted agonist/antagonist system was used. CH7C17 cells, a Jurkat T-cell derivative expressing the HA1.7 TCR, were stimulated with APCs loaded with an antagonist (HAK316A) or an agonist peptide (HA307-319), and the formation of ISs was examined. As for the OT-I system, the APA1/1 antibody, more than anti-CD3ζ antibody, made a clear distinction between synapses formed by agonist and antagonist peptides (Figure 5A-B). In this HA1.7 TCR system, the HAK316A peptide did not induce CD69 expression, unlike the agonist peptide (Figure 5C), but it was clearly not a “null” agonist because it elicited TCR translocation to the IS (Figure 5A-B) and tyrosine phosphorylation (data not shown).
APA1/1 demonstrates the conformational change in TCR in vivo
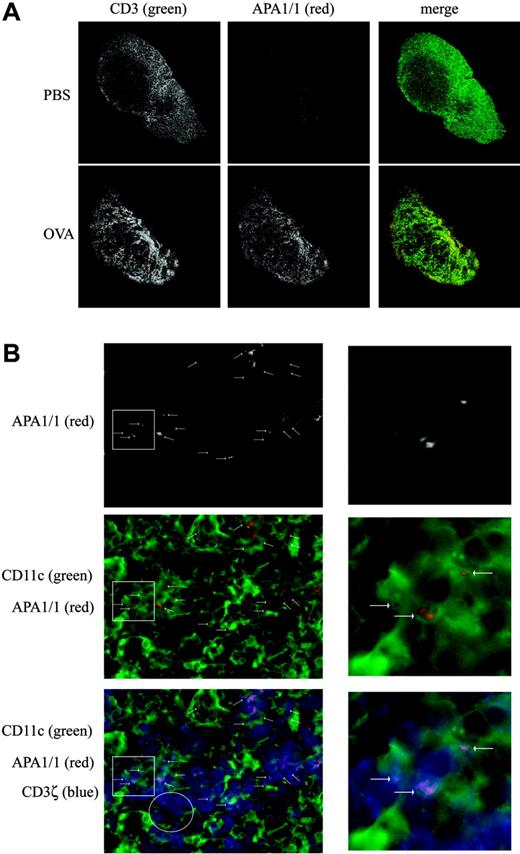
We finally studied whether the expression of the APA1/1 epitope could be detected in lymphoid tissue in vivo. Unlike a conventional anti-CD3 antibody, the APA1/1 antibody poorly stained lymph node sections of OT-I mice injected with vehicle alone (Figure 6A; PBS). Indeed, most T cells of nonimmunized TCR transgenic and nontransgenic mice were not recognized by the APA1/1 antibody (data not shown). However, when OT-I mice were subcutaneously injected with OVA, the draining lymph nodes showed a large increase in APA1/1 immunoreactivity as early as 6 hours after injection (Figure 6A; OVA). The total fluorescence signal within the lymph nodes (Figure 6A) was quantified in the green (conventional CD3) and red (APA1/1) channels using the ImageJ program. Although the fluorescence corresponding to the anti-CD3 antibody increased only 10% after OVA injection, APA1/1 fluorescence increased 300%. At higher magnifications, it was apparent that the APA1/1 antibody labeled almost two thirds (60%) of the OT-I T cells after OVA injection. Using CD11c as a marker for DCs, we found that OT-I T cells formed frequent APA1/1-positive synapses with these APC types (Figure 6B). The APA1/1 epitope was always distributed in a polarized manner (n = 199), clearly labeling the ISs formed with APCs bearing the H-2Kb-OVAp complex, as demonstrated by 25-D1.16 antibody staining (data not shown). Furthermore, most (81%) APA1/1-positive T cells formed synapses with APCs.
APA1/1 epitope distinguishes between synapses of CD4+T cells stimulated with agonist and with antagonist peptides. (A) T-cell/APC synapses. CH7C17 cells were incubated for 15 minutes with DAP-DR1-ICAM APCs loaded with 10 μM of the indicated peptides. DIC image of a representative T-cell/APC conjugate is shown for each condition. T cells were stained after fixation and permeabilization with anti-CD3ζ (green) and APA1/1 (red). Images were acquired with a Zeiss Axiovert 200 microscope with a CCD camera using a 100×/1.3 oil iris Plan-Neoplanar lens. (B) Quantitation of T-cell/APC synapses. Fifty to 60 T-cell/APC conjugates were counted per stimulation condition, and the percentage of cells with CD3ζ or APA1/1 concentrated in the IS is indicated as the mean ± SD of an experiment in duplicate. (C) T-cell stimulation. CH7C17 cells were stimulated for 24 hours with DAP-DR1-ICAM cells preloaded with the indicated concentrations of the agonist and antagonist peptides; induction of CD69 expression was evaluated by flow cytometry. Expression of CD69 is shown as the mean ± SD of an experiment in triplicate.
APA1/1 epitope distinguishes between synapses of CD4+T cells stimulated with agonist and with antagonist peptides. (A) T-cell/APC synapses. CH7C17 cells were incubated for 15 minutes with DAP-DR1-ICAM APCs loaded with 10 μM of the indicated peptides. DIC image of a representative T-cell/APC conjugate is shown for each condition. T cells were stained after fixation and permeabilization with anti-CD3ζ (green) and APA1/1 (red). Images were acquired with a Zeiss Axiovert 200 microscope with a CCD camera using a 100×/1.3 oil iris Plan-Neoplanar lens. (B) Quantitation of T-cell/APC synapses. Fifty to 60 T-cell/APC conjugates were counted per stimulation condition, and the percentage of cells with CD3ζ or APA1/1 concentrated in the IS is indicated as the mean ± SD of an experiment in duplicate. (C) T-cell stimulation. CH7C17 cells were stimulated for 24 hours with DAP-DR1-ICAM cells preloaded with the indicated concentrations of the agonist and antagonist peptides; induction of CD69 expression was evaluated by flow cytometry. Expression of CD69 is shown as the mean ± SD of an experiment in triplicate.
Hence, it appears that the APA1/1 epitope is exposed in CD8+ T cells in the lymph node when they encounter their nominal antigen (Figure 6), permitting the IS formation to be labeled in vivo. Furthermore, these results suggest that the APA1/1 antibody can be used to track and distinguish T cells whose TCRs are being engaged by fully stimulatory ligands in vivo, thus undergoing conformational change in vivo.
Discussion
In this study, we show that an epitope in the tail of CD3ϵ defined by APA1/1 is exposed when the TCR is engaged by stimulatory antibodies or MHCp. Furthermore, we show that the exposure of this epitope is rapid, takes place at 0°C, and is independent of PTK activity. Therefore, we conclude that exposure of the APA1/1 epitope is a hallmark of a conformational change in the TCR, similar to the Nck-binding assay (GST-Nck pull-down).15 Using CD8+ T cells from OT-I TCR transgenic mice as a model system, we show that the APA1/1 epitope is displayed when the TCR is engaged by a full agonist but not by a partial agonist/antagonist. This result adds an important control of specificity to the assay and suggests that the conformational change in the TCR is decisive for productive T-cell activation. In accordance with this idea, APA1/1 labels a restricted area of the cSMAC when formed with an APC bearing the full agonist MHCp and not the partial agonist. This restricted exposure of the APA1/1 epitope in the cSMAC suggests that only a fraction of the TCR in the IS undergoes the conformational change at any given time point. Finally, we demonstrate that in most resting T cells in the lymph node, the APA1/1 epitope is not accessible to the antibody; however, shortly after antigen exposure in vivo, it becomes exposed in most antigen-specific T cells. This result indicates that APA1/1 can be a useful tool to distinguish T cells whose TCR is being engaged by MHCp in vivo. Furthermore, the results demonstrate that the conformational change in the TCR occurs on antigen recognition in vivo.
Traditionally, it has been thought that the differential capacity of antibodies to detect specific epitopes reflects conformational changes. One of the most intensely studied examples of this is the increased affinity of integrins from inside-out signaling, promoting the exposure of neoepitopes in the alpha and beta subunit ectodomains.25,26 However, perhaps mechanistically, a more related example is that of the insulin receptor whose binding to insulin causes a conformational change in the associated G(i)α subunit, resulting in the exposure of a C-terminal epitope.27 Experimental evidence provided here confirms our previous work15 showing that TCR engagement results in the exposure of the proline-rich sequence in the tail of CD3ϵ responsible for Nck binding. Both assays, Nck binding and APA1/1 antibody recognition, reflect the same molecular event in different ways, that is, a rearrangement of the tail of CD3ϵ in the region of the proline-rich sequence. Nevertheless, rearrangement of the tail of CD3ϵ may only be part of the structural changes resulting from TCR engagement. In this regard, it would be interesting to determine whether other epitopes in the CD3 subunits become exposed on TCR engagement. Thus, though we linked the TCR conformational change to the remodeling of the actin cytoskeleton through the direct recruitment of Nck to CD3ϵ,15 other signaling pathways may also be modulated by the change in TCR conformation.
Detection of T cells whose TCR is being engaged by MHCp in vivo. (A) Increased APA1/1 reactivity on encountering the antigen in vivo. Draining lymph nodes from OT-I mice injected subcutaneously with 400 μg OVA or vehicle alone (PBS) were collected 6 hours after injection, fixed, and immunostained with both the anti-CD3 antibody 145-2C11 and APA1/1. Low-power magnification (5 ×) of the whole lymph node is shown. Total MFIs for green and red channels in 3 experiments were 67 318.5 ± 6824 (CD3, PBS), 74 371 ± 1839 (CD3, OVA), 25 011 ± 6237 (APA1/1, PBS), and 74 223 + 9658 (APA1/1, OVA). (B) APA1/1 staining is concentrated at the IS. High-power magnification (100 ×) of the lymph node from OVA-injected mice shown in panel A, illustrating that APA1/1 reactivity concentrates at the T-cell/APC contact sites (arrows). T cells are labeled with anti-CD3ζ in blue, APA1/1 in red, and APCs bearing the CD11c marker (DCs) in green. Higher magnifications of areas rich in APA1/1-positive synapses (white square insets) are shown to the right. The white circle inset denotes a T-cell-rich area (stained with anti-CD3ζ) in which there are no APA1/1-positive synapses. In 81% of the APA1/1+ cells, the APA1/1 epitope was exposed at a T-cell/APC contact site where the APC bears the H-2Kb/OVA complex (n = 199). Although 36% of the lymph node T cells were estimated to express the OT-I TCR by flow cytometry, 21% of the OVA-exposed lymph node T cells were APA1/1 positive (n = 369). Images were acquired with a Zeiss Radiance 2000 microscope using a 63×/1.4 oil Plan-Apochromatic lens.
Detection of T cells whose TCR is being engaged by MHCp in vivo. (A) Increased APA1/1 reactivity on encountering the antigen in vivo. Draining lymph nodes from OT-I mice injected subcutaneously with 400 μg OVA or vehicle alone (PBS) were collected 6 hours after injection, fixed, and immunostained with both the anti-CD3 antibody 145-2C11 and APA1/1. Low-power magnification (5 ×) of the whole lymph node is shown. Total MFIs for green and red channels in 3 experiments were 67 318.5 ± 6824 (CD3, PBS), 74 371 ± 1839 (CD3, OVA), 25 011 ± 6237 (APA1/1, PBS), and 74 223 + 9658 (APA1/1, OVA). (B) APA1/1 staining is concentrated at the IS. High-power magnification (100 ×) of the lymph node from OVA-injected mice shown in panel A, illustrating that APA1/1 reactivity concentrates at the T-cell/APC contact sites (arrows). T cells are labeled with anti-CD3ζ in blue, APA1/1 in red, and APCs bearing the CD11c marker (DCs) in green. Higher magnifications of areas rich in APA1/1-positive synapses (white square insets) are shown to the right. The white circle inset denotes a T-cell-rich area (stained with anti-CD3ζ) in which there are no APA1/1-positive synapses. In 81% of the APA1/1+ cells, the APA1/1 epitope was exposed at a T-cell/APC contact site where the APC bears the H-2Kb/OVA complex (n = 199). Although 36% of the lymph node T cells were estimated to express the OT-I TCR by flow cytometry, 21% of the OVA-exposed lymph node T cells were APA1/1 positive (n = 369). Images were acquired with a Zeiss Radiance 2000 microscope using a 63×/1.4 oil Plan-Apochromatic lens.
Our results show that the conformational change in the TCR is provoked by full agonists and not by partial agonists. Thus, it would be interesting to determine whether known molecular correlates of TCR signaling induced by antagonist peptides—partial phosphorylation of CD3ζ to the p21 form, recruitment but not phosphorylation of ZAP70, and, most important, poor CD3ϵ phosphorylation8,9 —are related to the failure of the TCR to modify its conformation. It is possible that TCR engagement induces a general rearrangement of the CD3 tails, exposing them to complete tyrosine phosphorylation by Lck. The failure to unmask the CD3 tails would impair Lck-mediated phosphorylation when T cells are stimulated with an antagonist peptide and might be reflected in a failure to recruit Lck to antagonist peptide-induced IS.7 Nevertheless, if the TCR conformational change affects only the tail of CD3ϵ, we could envision Nck recruitment, together with tyrosine phosphorylation of CD3ϵ and CD3ζ, as among the primary events impaired by antagonist peptide binding to the TCR.
Antagonist peptides have been shown to form abnormal ISs in which the TCR3 and MHCp2 are less densely accumulated than in ISs formed by full agonists. Furthermore, antagonist peptides seem to impair the recruitment of Lck to the synapse,7 which, on the other hand, correlates with an inhibition in the TCR-CD4 interaction in the IS.6 Interestingly, the conformational change in the TCR was initially proposed to explain cocapping of CD4 induced by anti-CD3 antibodies.28 Indeed the findings of Gascoigne and colleagues6 may indirectly show that antagonist peptides fail to induce the conformational change in the TCR and the TCR-CD4 interaction in the IS. Thus, antagonist peptides do not produce the 2 effects thus far described that are indicative of a conformational change in the TCR: CD4 cocapping and exposure of the proline-rich sequence in CD3ϵ.
How recognition of a full agonist or an antagonist ligand is reflected in the differential exposure of the CD3ϵ tail remains unclear. The crystal structure of the soluble A6 TCRα/β ectodomain associated with soluble HLA-A2 bound to agonist and antagonist peptides13 does not reveal structural differences indicative of a conformational change in the TCRα/β ectodomain that might enable it to distinguish between ligands. This has been confirmed, with one exception,29 in other studies in which no structural rearrangements of the TCRα/β ectodomain have been observed beyond the induced fit of the CDR3 loops.14,30 We favor a multimeric TCR model to explain the transmission of a conformational change to the CD3ϵ tail without modifying the TCRα/β structure. Although recent in vitro data suggest that the TCR complex is monovalent (it contains only one ligand-binding TCRα/β heterodimer),31 ample evidence in vivo has shown that the TCR complex may contain at least 2 TCRα/β heterodimers.18,32,33 More recently, we have shown that the TCR coexists as a mixture of monovalent and multivalent complexes.37 We postulate that the simultaneous binding of dimeric MHCp to 2 TCRα/β ectodomains within the same complex would modify the TCRα/β-CD3 interactions (through the CD3 ectodomains, transmembrane domains, or both) thus “pushing” the CD3 dimers. This modification would result in the transmission of the conformational change to the tail of CD3ϵ. Differences in the biologic effects of full agonists and antagonists have been proposed as relying largely on kinetic parameters: antagonist ligands bind with lower affinities and have higher dissociation rates.34 The conformational change in the TCR could represent a direct interpretation of the ligand-binding kinetics. Thus, depending on the affinity and the dissociation rates, bivalent binding of MHCp to the TCR could be converted to a monovalent interaction that cannot exert a push on the CD3 dimers and, therefore, cannot induce conformational change in the TCR. This model might explain how significant binding of an H-2Kb/Ig dimer complexed to the antagonist E1 can be observed without exposing the APA1/1 epitope (Figure 2C). What the model does not explain, however, is how the response to agonist and antagonist peptides in the thymus can be so different to the periphery. Indeed, our data suggest that E1, which acts as a positive-selecting ligand in OT-I thymocytes,16 induces the conformational change in the TCR as efficiently as OVAp.35 Our current hypothesis is that the TCR in thymocytes responds more efficiently to weak ligands because it differs from the TCR in mature T cells. One possibility is that differences in posttranslational modifications that occur as T cells mature36 make the TCR less prone to transduce the conformational change.
Recently, DCs were shown to form ISs with T cells in the absence of antigen, and it was proposed that this interaction could lead to the activation of signaling pathways involved in the maintenance of the naive T cell pool in vivo.24 In this work, the translocation of the TCR to the T-cell/DC synapse was indeed detected in the absence of antigen. In light of our ex vivo experiments with antagonist and agonist peptides, one might anticipate that APA1/1 would not be exposed in the T-cell/DC synapses in which an appropriate MHCp was not engaged and in which the TCR would not undergo a conformational change. This further supports the notion that the conformational change in the TCR is required for full activation.
Finally, the results described here suggest that the APA1/1 antibody can be used as a tool to track productively engaged T cells in vivo. The antibody could, for example, be used in histochemical studies to identify a correlation between the induction of a conformational change in the TCR of infiltrating T cells and tumor or allograft rejection.
Prepublished online as Blood First Edition Paper, March 24, 2005; DOI 10.1182/blood-2004-12-4763.
Supported by grants from Comisión Interministerial de Ciencia y Tecnología (SAF2002-03589) (B.A.), Comunidad de Madrid 08.3/0030/2001 (B.A.), Formación de Profesorado Universitario Fellowship (R.M.R.), and Fundación Areces (to the CBMSO).
An Inside Blood analysis of this article appears in the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank C. Ardavín, B. Cubelos, M. Mittelbrunn, and E. Palmer for kindly providing reagents and protocols and A. Borroto, S. Ley, H. M. van Santen, and M. Sefton for critically reading the manuscript. We also thank T. Cerrato, M. A. Muñoz, and T. Villalba for their excellent technical support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal