Abstract
Glucocorticoids (GCs) play an important role in the regulation of peripheral T-cell survival. Their molecular mechanism of action and the question of whether they have the ability to inhibit apoptosis in vivo, however, are not fully elucidated. Signal transduction through the glucocorticoid receptor (GR) is complex and involves different pathways. Therefore, we used mice with T-cell-specific inactivation of the GR as well as mice with a function-selective mutation in the GR to determine the signaling mechanism. Evidence is presented for a functional role of direct binding of the GR to 2 negative glucocorticoid regulatory elements (nGREs) in the CD95 (APO-1/Fas) ligand (L) promoter. Binding of GRs to these nGREs reduces activation-induced CD95L expression in T cells. These in vitro results are fully supported by data obtained in vivo. Administration of GCs to mice leads to inhibition of activation-induced cell death (AICD). Thus, GC-mediated inhibition of CD95L expression of activated T cells might contribute to the anti-inflammatory function of steroid drugs. (Blood. 2005;106:617-625)
Introduction
Upon triggering of their T-cell receptor (TCR), resting naive T lymphocytes are activated to elicit an immune response. In contrast, preactivated T cells undergo activation-induced cell death (AICD) in response to the same signal.1-3 Apoptosis by AICD is an important mechanism to down-regulate the number of reactive T cells and to terminate the immune response.4 The main apoptotic pathway of AICD is mediated by the CD95 (apoptosis antigen-1 [APO-1]/Fas) death receptor system since cell death by TCR restimulation can be blocked almost completely in the presence of reagents that inhibit CD95/CD95L interaction.1-3 CD95 ligand (CD95L, CD178) is induced upon TCR/CD3 cross-linking and triggers CD95 signaling and subsequently apoptosis.5 Deregulation of CD95L expression results in several diseases, such as autoimmunity and uncontrolled lymphoproliferation.6-8 The transcriptional regulation of the CD95L promoter upon TCR/CD3 stimulation has been studied extensively. Various transcription factors and regulatory elements have been identified, such as several binding sites for nuclear factor of activated T cells (NF-AT), nuclear factor κB (NF-κB), activator protein 1 (AP-1), early growth response (Egr) protein, and interferon regulatory factor 1 (IRF-1).9
The glucocorticoid receptor (GR) is a ligand-dependent transcription factor that acts as a major modulator of the immune system due to its anti-inflammatory and immunosuppressive activities, thus serving a function that is frequently put to use in the treatment of autoimmune disorders, inflammatory diseases, and allergy. In the absence of ligand, the receptor is retained in the cytoplasm in an inactive state forming a large complex that includes chaperones.10 Upon hormone binding, the GR dissociates from the cytoplasmic complex and translocates into the nucleus. Within the nucleus it acts as a transcriptional activator by binding as a homodimer with high affinity to glucocorticoid responsive DNA elements (GREs). In addition to enhancing the rate of transcription, the GR can also act as a transcriptional repressor by binding to different DNA sequences called negative GREs (nGREs).11,12 Besides direct DNA binding, the GR can also modulate transcription as a monomer via protein-protein interactions as documented by repressive effects on AP-1 and NF-κB13-15 and activation of signal transducer and activator of transcription 5 (Stat-5).16
Glucocorticoid (GC) hormones exert their potent anti-inflammatory action mainly by inhibition of cytokine gene expression, and hence effectively block the activation of the immune system.15,17,18 In addition, GCs are potent inducers of apoptosis in thymocytes and in some T-cell hybridomas by DNA binding-dependent transcriptional regulation, whereas peripheral T cells are only weakly sensitive toward GC-induced cell death. Although both GC and TCR stimulation can induce apoptosis in T-cell hybridomas, surprisingly, cell death is strongly reduced when both stimuli are provided simultaneously.19 However, the actual molecular mechanism of how GCs inhibit T-cell apoptosis remains unclear so far.20-22
Using mice with a T-cell-specific inactivation of the GR as well as mice that carry a point mutation within the dimerization region of the GR (GRdim), we have been able to demonstrate that the antiapoptotic effect of GCs on AICD is due to direct binding of the GR to 2 newly identified nGREs in the CD95L promoter, thereby repressing its transcriptional activation in peripheral T cells. Moreover, we can show that the inhibition of AICD is not dependent on protein synthesis of transcriptional regulators and that a monomeric GR is not sufficient for mediating the antiapoptotic effect of GCs.
Materials and methods
Generation of mutant mice by gene targeting
GRloxP mice were generated by homologous recombination in ES cells as described previously.23 LckCre transgenic mice were generated by oocyte injection as described in Orban et al.24 To generate GRlckCre mice, we crossed GRlckCre/+ mice with GRloxP/loxP mice and used resulting GRloxP/loxP; lckCre (GRlckCre) mice as the conditional T-cell-specific mutant mice. In this case, controls were of the genotype GRloxP/loxP. The GRdim mice have been described previously.25 All mice were kept under specific pathogen-free conditions in the central animal facility of the DKFZ Heidelberg, Germany.
Isolation, culture, and activation of peripheral murine T cells
Single-cell suspensions from spleen and mesenteric lymph nodes were prepared by standard procedures. Briefly, the organs were removed from the mice, disrupted, and filtered through a 40-μm cell strainer (Falcon, Bedford, MA). Cells were further purified from erythrocytes using ammonium chloride ACK lysis buffer (0.155 M NH4Cl, 0.01 M KHCO3, and 0.1233 M EDTA [ethylenediaminetetraacetic acid, pH 7.27]); from adhering monocytes and macrophages by monodepletion for 1 hour in a cell-culture bottle; and from B cells by attaching them for 2 hours to a cell-culture plate coated for 3 hours at 37°C with goat anti-mouse immunoglobulin (Ig) (heavy [H] and light [L]) antibody (Biozol, Eching, Germany). The purity of T lymphocytes obtained by this procedure was found to be more than 90% by flow cytometry analysis. T cells were stimulated with 5 μg/mL concanavalin A (ConA) (Pharmazia, Uppsala, Sweden) for 16 hours, and subsequently washed 3 times with phosphate-buffered saline (PBS). Cells were maintained for an additional 4 days (day-5 T cells) in RPMI medium containing 10% fetal calf serum (FCS), 50 μM 2-mercaptoethanol, 2 mM glutamine, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 50 μg/mL gentamycin, and 25 U/mL interleukin-2 (IL-2). The murine hybridoma T-cell clone DC27.1 was described previously.26
Preparation of human T cells from peripheral blood
Human peripheral T cells were prepared as described.27 For activation, resting T cells were cultured at 2 × 106 cells/mL with 1 μg/mL phytohemagglutinin (PHA) for 16 hours. T cells were then washed 3 times and cultured for an additional 5 days (day-6 T cells) in the presence of 25 U/mL IL-2.
Cell analysis by flow cytometry
Single-cell suspensions were incubated for 15 minutes on ice with a 1:100 dilution of the appropriate antibody in 50 μL PBS. The following R-phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, and Cychrome-conjugated monoclonal antibodies (Pharmingen, San Diego, CA) were used: 145-2C11 (anti-mouse CD3), H129.19 (anti-mouse CD4), 53-6.7 (anti-mouse CD8), 7D4 (anti-mouse CD25), H1.2F3 (anti-mouse CD69), Jo2 (anti-mouse CD95), H57-597 (anti-mouse TCRβ), and RA3-6B2 (anti-mouse B220). For CD95L cell-surface staining, cells were stained with a biotinylated anti-CD95L antibody (Ab, MFL3) and, in a second step, streptavidin-CyChrome. A biotinylated hamster IgG Ab (A19-3) was used as an isotype control for MFL3. To control the specificity of the CD95L mAb, the melanoma cell line B16 stable transfected with either empty vector or muCD95L was used (data not shown). The 3-color cytofluorometric analysis was performed on a FACScan Cytometer (Becton Dickinson, Mountain View, CA) using Cell Quest Software (Becton Dickinson). A minimum of 10 000 cells per sample was analyzed.
Induction of apoptosis
Anti-CD95 stimulation. Recombinant cytotoxic CD95L, trimerized via a modified leucine-zipper domain (LZ-CD95L), which binds both to human and murine CD95,28,29 was added as supernatant to cell cultures at the indicated dilutions.
Anti-CD3 treatment. Cell-culture plates were coated with anti-CD3 mAb (10 μg/mL 145-2C11 for murine cells; 50 μg/mL OKT3 for human cells) for 3 hours at 37°C or overnight at 4°C and washed with PBS.
Dexamethasone treatment. The synthetic glucocorticoid dexamethasone (Dex; Sigma, Taufkirchen, Germany) was added to the culture medium at the indicated final concentrations. Mifepristone (RU486), cycloheximide (CHX), and actinomycine D (ActD) were obtained from Sigma.
Determination of apoptosis
For cytofluorimetric evaluation of apoptosis, the cells were treated with propidium iodide at a final concentration of 2.5 μg/mL and directly analyzed by FACScan. Dead cells are propidium iodide positive. Alternatively, cell death was determined by a drop in the forward scatter (FSC)/side scatter (SSC) profile or by measurement of DNA fragmentation according to the method of Nicoletti et al.30 Specific apoptosis was calculated as follows: (percentage of experimental apoptosis - percentage of spontaneous apoptosis)/(100 - percentage of spontaneous apoptosis) × 100.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using TRIzol RNA purification kit (Gibco, Carlsbad, CA). cDNA synthesis was performed with GeneAmp-kit (Perkin Elmer, Warrington, United Kingdom). cDNA (1 μg) was used for PCR using High Fidelity PCR Master kit (Roche, Mannheim, Germany). PCR was carried out for 30 cycles (94°C 1 minute; 60°C 1 minute; 72°C 1 minute) with the following primers (Sigma ARK, Darmstadt Germany): beta-actin forward, 5′ ATT GTT ACC AAC TGG GAC GAC ATG 3′ and beta-actin reverse, 5′ CTT CAT GAG GTA GTC TGT CAG GTC 3′; glucocorticoid-induced leucine-zipper (GILZ) forward, 5′ CTC TCA ACC CCA TTC TAT GTA G 3′ and GILZ reverse, 5′ GGC AGA GAT GGG AGA GAT TG 3′; CD95L forward, 5′ TTC ACT CCA GAG ATC AGA GCG G 3′ and CD95L reverse, 5′ CAG CAG TGC CAC TTC ATC TTG G 3′; and B-cell lymphoma-2 (bcl-2) forward, 5′ CGA CTT CGC CGA GAT GTC CAG CCA G 3′ and bcl-2 reverse, 5′ ACT TGT GGC CCA GAT AGG CAC CCA G 3′. PCR products were analyzed by agarose gel electrophoresis.
In vivo mouse model for AICD
Anti-CD3 antibody (5 μg, 145-2C11; Pharmingen) was injected intravenously into the tail vein of 8-week-old C57BL/6 mice. Then, 20 hours later the spleen was removed from the animals and the T-cell-to-B-cell ratio was determined by fluorescence-activated cell sorter (FACS) analysis. Mice were pretreated for 24 hours either with 300 μg/L dexamethasone-phosphate (Sigma) in the drinking water or water only as control. An amount of 300 μg/L leads to an average uptake of about 1.35 μg/d per mouse, which, when calculated to the body weight, fits to the low-dose therapeutic range of oral GC treatment of patients.31,32 This concentration does not induce peripheral T-cell or B-cell apoptosis in vivo (data not shown).
ABCD assay
The ABCD (avidin, biotin, complex, DNA) assay is based on immobilization of protein-DNA complexes via binding of a biotinylated oligonucleotide to a streptavidin matrix. A total of 200 μL Jurkat whole-cell extract (4.5 μg/μL) was incubated with 200 μL buffer H (100 mM KCl, 20 mM HEPES [pH 7.8], 20% glycerol, 1 mM dithiothreitol [DTT], 0.1% nonidet P40 [NP40]), 2 μg biotinylated oligo, and biotinylated PCR product, respectively, 10 μg herring sperm DNA and Dex for 1 hour on ice. After addition of 50 μL equilibrated streptavidin agarose beads (Pierce, Rockford, IL), incubation was continued for 30 minutes at 4°C on a rotator. Beads were washed repeatedly with buffer H (containing 50 mM KCl) and boiled in Laemmli sample buffer, and proteins were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. GR was detected by Western blot (anti-GR antibody H300; Santa Cruz Biotechnology, Santa Cruz, CA). Enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ) was used for detection. Genomic DNA (120 ng) from AtT20 cells was used for PCR (Platinum Pfx DNA polymerase; Invitrogen, Frederick, MD). PCR was carried out for 30 cycles (94°C for 1 minute; 68°C for 2 minutes) using the following primers: The fragments from -765 to -624 and from -624 to -544 were obtained using the primers 5′ GAG TGG TCG GTT TTA TAA AGG C 3′ and 5′ GGA AAT GTA GCC TTC CTC CTC T 3′ followed by digestion with Hpy188I (cuts at -624). The fragment from -561 to -444 was obtained using the primers 5′ AGA GGA GGA AGG CTA CAT TTC C 3′ and 5′ AAC ATC AGC CAG CTG CAA C 3′. The fragments from -135 to +122 and from +122 to +225 were obtained using the primers 5′ GTG GGT GTC TCA CAG AGA AGC 3′ and 5′ GAC ACA GGT GGT GGT GGA G 3′ followed by digestion with BglII (cuts at +122). Biotinylated PCR products were analyzed by agarose gel electrophoresis prior to using them for the ABCD assay. Biotinylated oligos were from ThermoHybAid (Ulm, Germany) and were composed of the following sequences: FasL-700, 5′ TGA ATA ATG TTT TAG TAT GTG CTG TGT GAT G 3′; FasL-600, 5′ CCT CAG TTT TCA TCT GGT GAC CAG AAG AGA G 3′; FasL-500, 5′ GGG GTT AGG GCA GCC TTG AAC ACC TGG CAC ACA TTC CTG 3′; FasL-700 mut, 5′ TGA ATA ATG ATT TAG TAT CTG CTG TGT GAT G 3′; FasL-500 mut, 5′ GGG GTT AGG GCA GCC TTG AAG ACC TGG CAT ACA TTC CTG 3′; TAT, 5′ AAT CTC TGC TGT ACA GGA TGT TCT AGC TAC T 3′; and TATmut, 5′ AAT CTC TGC TGT AGA GGA TCT TCT AGC TAC T 3′.
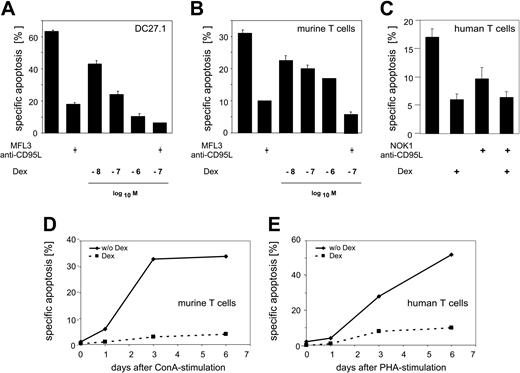
GCs inhibit AICD in primary peripheral T lymphocytes. (A) DC27.1 T cells were stimulated by anti-CD3 antibody for 18 hours in the presence or absence of the indicated concentrations of Dex or of the antagonistic anti-CD95L antibody MFL3 (10 μg/mL). (B) Same as in panel A, but using primary murine T cells 5 days after initial stimulation with ConA (day-5 T cells). (C) Same as in panel A, but using primary human T cells 6 days after initial stimulation with PHA (day-6 T cells), 10-7 M Dex, and the antagonistic anti-CD95L antibody NOK1 (10 μg/mL). Error bars represent mean ± SEM. (D-E) Primary murine (D) and human (E) T cells were restimulated by anti-CD3 antibody for 18 hours in the presence or absence of 10-7 M Dex at the indicated time point after initial T-cell stimulation. Apoptosis was determined by FACS analysis (FSC/SSC method for A-C and the Nicoletti et al30 method for D-E). Results are representative of at least 3 independent experiments.
GCs inhibit AICD in primary peripheral T lymphocytes. (A) DC27.1 T cells were stimulated by anti-CD3 antibody for 18 hours in the presence or absence of the indicated concentrations of Dex or of the antagonistic anti-CD95L antibody MFL3 (10 μg/mL). (B) Same as in panel A, but using primary murine T cells 5 days after initial stimulation with ConA (day-5 T cells). (C) Same as in panel A, but using primary human T cells 6 days after initial stimulation with PHA (day-6 T cells), 10-7 M Dex, and the antagonistic anti-CD95L antibody NOK1 (10 μg/mL). Error bars represent mean ± SEM. (D-E) Primary murine (D) and human (E) T cells were restimulated by anti-CD3 antibody for 18 hours in the presence or absence of 10-7 M Dex at the indicated time point after initial T-cell stimulation. Apoptosis was determined by FACS analysis (FSC/SSC method for A-C and the Nicoletti et al30 method for D-E). Results are representative of at least 3 independent experiments.
ChIP assay
The chromatin immunoprecipitation (ChIP) protocol was adopted from Metivier et al.33 DC27.1 cells, cultivated in RPMI containing 10% charcoal-stripped bovine calf serum, were incubated with 10-7 M Dex for 30 minutes, cross-linked by paraformaldehyde (PFA, 1%) for 10 minutes at room temperature. The reaction was stopped with 0.125 M glycine. Cells were washed and lysates were subjected to immunoprecipitation with anti-GR antibodies (Santa Cruz Biotechnology) using buffers described by Metivier et al.33 Immunoprecipitated material was collected by centrifugation and washed twice with radioimmunoprecipitation assay (RIPA), once with WBI, once with WBII supplemented with 500 mM NaCl, once with LiCl buffer (0.25 M LiCl, 0.5% deoxycholate [DOC], 0.5% NP40, 1 mM EDTA, 0.5 mM EGTA [ethylene glycol tetraacetic acid], 20 mM HEPES [pH 8.5]), and twice with Tris-EDTA (TE) buffer. A total of 200 μL digestion buffer (50 mM Tris [tris(hydroxymethyl)aminomethane, pH 8], 1 mM EDTA, 100 mM NaCl, 0.5% SDS) supplemented with proteinase K (100 μg/mL) was added, and the reaction was incubated at 55°C for 3 hours. The material was de-cross-linked by raising the temperature to 65°C for 16 hours. Recovered DNA was purified by PCR purification kit (Qiagen, Valencia, CA) according to manufacturer's instructions. Of the obtained DNA, 2 μL was used in a 20-μL PCR reaction (Taq DNA Polymerase; Qiagen) using the following primers: FasL700F, 5′ CTG AAG TCA AAC CTA AGG CAG AG 3′; FasL700R, 5′ AGG TGC CTA ATT CAG AAG AGT TTG 3′; FasLstart, 5′ CAT GCA GCA GCC CAT GGA ATT ACC 3′; FasL+180, 5′ GAT GGC GGT GGT AGT GGT GAC 3′; FasintrF, 5′ CAG AGA TCT GAA ATT ATC CTC ATA ATC 3′; and FasintrR, 5′ GGC TCA CAC ACC TGA CAT GCA TC 3′.
Statistical analysis
Data are expressed as means plus or minus SEM. Comparisons were made using unpaired, 2-tailed Student t test and differences were considered significant if P was less than .01.
Results
GCs inhibit AICD in primary peripheral T lymphocytes
It has previously been shown that GCs such as dexamethasone (Dex) can interfere with TCR/CD3-induced apoptosis in T-cell hybridomas.20-22 To test this in other T-cell populations, we have induced apoptosis by stimulating the murine T-cell line DC27.1 and preactivated primary T cells from murine spleen or from human peripheral blood via plate-bound anti-CD3 antibody. The data shown in Figure 1 demonstrate a dose-dependent antiapoptotic effect of Dex on AICD. The Dex concentrations used in these experiments do not induce apoptosis in T cells themselves (Figure 2A). Blocking with a CD95L antagonistic antibody confirms the importance of the CD95 system in AICD (Figure 1A-C). Primary naive T cells are resistant toward TCR/CD3-mediated AICD. However, T cells become apoptosis sensitive upon stimulation and prolonged culture in IL-2-containing medium1,27 (Figure 1D-E). The presence of Dex during TCR/CD3 restimulation significantly reduced AICD at every time point investigated (Figure 1D-E). These data demonstrate that GCs capable of inducing apoptosis in a variety of cell types at high doses, at low to moderate concentrations, have an antiapoptotic potential on AICD of primary human and murine T lymphocytes.
GC treatment does not affect CD95 signaling but regulates CD95L expression
To further investigate which steps in the AICD signaling pathway are negatively modulated by GCs, we have analyzed the sensitivity toward cell death induced by direct triggering of the CD95 death receptor via its ligand using LZ-CD95L as stimulator. The results presented in Figure 2A show that the pathway downstream of CD95 is not blocked. It has been suggested that GC treatment up-regulates Bcl-2 in T cells and thereby protects them from CD95-induced apoptosis.34 However, we did not observe any change in Bcl-2 mRNA expression in primary murine T cells (Figure 3B). In addition, expression of CD95, TCRβ, and CD3ϵ on the cell surface was not altered by Dex treatment (data not shown). These results suggest that during the process of AICD, Dex acts upstream of the CD95 death receptor.
GC treatment does not affect CD95 signaling but regulates CD95L expression. (A) Primary murine day-5 T cells (left) and human day-6 T cells (right) were treated for 18 hours with the indicated concentrations of LZ-CD95L in the presence or absence of the indicated concentrations of Dex. Apoptosis was determined by propidium iodide-FACS analysis. (B) Murine day-5 T cells (left) and DC27.1 T cells (right) were (re-) stimulated by anti-CD3 antibody in the presence or absence of Dex (10-8, 10-7, and 10-6 M) for 8 hours. CD95L protein expression on the cell surface was assayed by FACS analysis. Of 5 experiments, 1 representative is shown. Inset graphs show histograms for CD95L staining.
GC treatment does not affect CD95 signaling but regulates CD95L expression. (A) Primary murine day-5 T cells (left) and human day-6 T cells (right) were treated for 18 hours with the indicated concentrations of LZ-CD95L in the presence or absence of the indicated concentrations of Dex. Apoptosis was determined by propidium iodide-FACS analysis. (B) Murine day-5 T cells (left) and DC27.1 T cells (right) were (re-) stimulated by anti-CD3 antibody in the presence or absence of Dex (10-8, 10-7, and 10-6 M) for 8 hours. CD95L protein expression on the cell surface was assayed by FACS analysis. Of 5 experiments, 1 representative is shown. Inset graphs show histograms for CD95L staining.
As shown in Figure 2B, TCR/CD3 stimulation leads to a strong induction of CD95L protein expression on the cell surface of primary murine T cells as well as on DC27.1 T cells. Addition of Dex clearly reduces activation-induced CD95L cell-surface protein expression in a dose-dependent manner (Figure 2B). These data demonstrate that GCs affect AICD by interfering with activation-induced CD95L expression.
Dex-induced protein synthesis is not required for inhibition of CD95L mRNA expression. (A) Primary murine day-5 T cells were restimulated by anti-CD3 antibody in the presence or absence of 10-7 M Dex for the indicated periods of time. The mRNA expression level of CD95L and β-actin was detected by RT-PCR. (B) Primary murine day-5 T cells were restimulated by anti-CD3 antibody in the presence or absence of 10-7 M Dex for 5 hours. The mRNA expression of CD95L, GILZ, Bcl-2, and β-actin was detected by RT-PCR. (C) Same as in panel B, but restimulation with or without pretreatment for 2 hours with 10 μg/mL CHX. The mRNA expression of CD95L and β-actin was detected by RT-PCR. Results are representative of at least 3 independent experiments.
Dex-induced protein synthesis is not required for inhibition of CD95L mRNA expression. (A) Primary murine day-5 T cells were restimulated by anti-CD3 antibody in the presence or absence of 10-7 M Dex for the indicated periods of time. The mRNA expression level of CD95L and β-actin was detected by RT-PCR. (B) Primary murine day-5 T cells were restimulated by anti-CD3 antibody in the presence or absence of 10-7 M Dex for 5 hours. The mRNA expression of CD95L, GILZ, Bcl-2, and β-actin was detected by RT-PCR. (C) Same as in panel B, but restimulation with or without pretreatment for 2 hours with 10 μg/mL CHX. The mRNA expression of CD95L and β-actin was detected by RT-PCR. Results are representative of at least 3 independent experiments.
A GR monomer is not sufficient to mediate the antiapoptotic effect of GCs in T cells
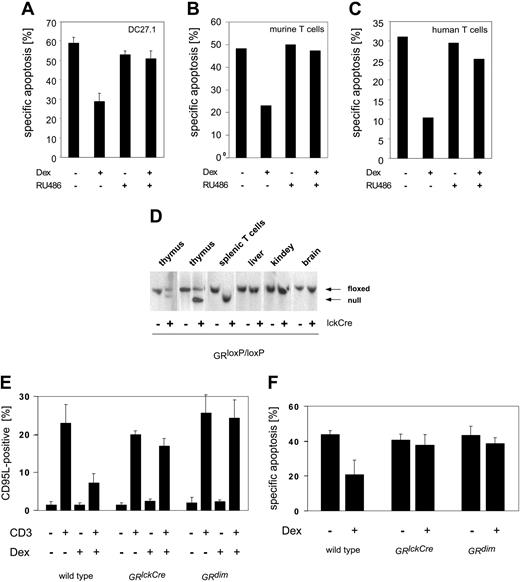
The GR is activated by binding of GCs. To exclude GR-independent effects mediated by Dex in T cells, we used the GR antagonist RU486 (Mifepristone). RU486 binds at the ligand-binding domain of GR and thereby prevents binding of coactivators to the GR, leading to inhibition of GR function.35 The antiapoptotic effect of Dex on AICD is reversed by RU486 treatment in DC27.1 T cells and in primary human and murine T cells (Figure 4A-C), demonstrating that this mechanism is GR dependent.
The GR can function either as a homodimeric transcription factor or, in a monomeric form, as a transcriptional regulator by direct interaction with other transcription factors. To investigate which of these pathways is involved in the modulation of AICD by GCs, we used primary T cells from 2 different GR mutant mouse strains. Using the Cre/loxP-system, T-cell-specific GR-deficient mice were generated (GRlckCre). These mice show a complete recombination of the GR gene locus (Figure 4D) and no GR protein expression (data not shown) in peripheral T cells. In addition, we used GRdim mice, which carry a mutation in the GR, preventing homodimerization and thereby blocking direct transactivation.25 Nevertheless, modulation of gene expression by affecting transcription factors such as AP-1 and NF-κB is not impaired in GRdim mice.14,15
Thymocyte development, T-cell proliferation, and apoptosis upon different stimuli are unchanged in GR-deficient compared with wild-type T cells (data not shown; Figure 4E). This is in agreement with studies using mice expressing a hypomorphic GR allele without an N-terminal transactivation domain.36-39
We have shown that TCR restimulation leads to induction of CD95L expression (Figure 4E). This effect is strongly reduced by GCs in T cells from wild-type mice. As expected, repression of TCR-dependent CD95L induction is absent in GR-deficient T cells of GRlckCre mice. Interestingly, this is also observed in T cells from mice (GRdim) with a dimerization defect GR (Figure 4E). In agreement with this finding, we do not observe a reduction of apoptosis by GCs in T cells of GRlckCre and GRdim mice (Figure 4F), demonstrating that rescue from AICD depends on the DNA-binding function of the GR. These data indicate that a GR monomer is not sufficient to mediate the antiapoptotic effect of GCs in T cells. Therefore, a direct interference with NF-κB or AP-1 can be excluded as a mechanism of inhibition.
A GR monomer is not sufficient to mediate the antiapoptotic effect of GCs in T cells. DC27.1 T cells (A), primary murine day-5 T cells (B), and primary human day-6 T cells (C) were (re-) stimulated by anti-CD3 antibody in the presence or absence of 10-7 M Dex and/or 5 μM RU486 for 18 hours. Apoptosis was determined by FSC/SSC-FACS analysis. Results are for at least 4 independent experiments. (D) Southern blot analysis of the GR gene locus. Thymocytes, peripheral splenic T cells, and cells from liver, kidney, and brain were tested for successful recombination in GRloxP/loxP mice with or without expression of Cre-recombinase under the control of the lck-promoter (GRlckCre). Upper band: loxP-flanked locus (floxed); lower band: recombined locus (null). (E) Day-5 T cells from GRlckCre, GRdim, or control mice (GRloxP/loxP) were restimulated by anti-CD3 antibody for 8 hours in the presence or absence of 10-7 M Dex. CD95L protein expression on the cell surface was assayed by FACS analysis (n = 4). (F) Same as in panel E, but determination of apoptosis after 18 hours of restimulation (n = 3). Error bars are mean ± SEM.
A GR monomer is not sufficient to mediate the antiapoptotic effect of GCs in T cells. DC27.1 T cells (A), primary murine day-5 T cells (B), and primary human day-6 T cells (C) were (re-) stimulated by anti-CD3 antibody in the presence or absence of 10-7 M Dex and/or 5 μM RU486 for 18 hours. Apoptosis was determined by FSC/SSC-FACS analysis. Results are for at least 4 independent experiments. (D) Southern blot analysis of the GR gene locus. Thymocytes, peripheral splenic T cells, and cells from liver, kidney, and brain were tested for successful recombination in GRloxP/loxP mice with or without expression of Cre-recombinase under the control of the lck-promoter (GRlckCre). Upper band: loxP-flanked locus (floxed); lower band: recombined locus (null). (E) Day-5 T cells from GRlckCre, GRdim, or control mice (GRloxP/loxP) were restimulated by anti-CD3 antibody for 8 hours in the presence or absence of 10-7 M Dex. CD95L protein expression on the cell surface was assayed by FACS analysis (n = 4). (F) Same as in panel E, but determination of apoptosis after 18 hours of restimulation (n = 3). Error bars are mean ± SEM.
Dex-induced protein synthesis is not required for inhibition of CD95L expression in T cells
Inhibition of CD95L expression by Dex can also be observed at the mRNA level, showing that regulation of CD95L expression by GCs occurs on the level of transcription (Figure 3A). Many transcription factors are known to be involved in activation-induced up-regulation of CD95L, including AP-1 and NF-κB. GCs are capable of inducing the expression of the inhibitor-of-κB (IκB),40,41 an inhibitor of NF-κB. In addition, GCs lead to induction of the antiapoptotic molecule GILZ (glucocorticoid-induced leucine-zipper) (Figure 3B), a protein that can interfere with AP-1 and NF-κB activation.42-44 To test if induction of these molecules is involved in inhibition of AICD in primary T cells, we investigated if de novo protein synthesis (eg, of GILZ or IκB) is required. Therefore, activated T cells were pretreated for 2 hours with CHX to inhibit protein biosynthesis, and subsequently were restimulated for 5 hours with anti-CD3 antibody in the presence or absence of Dex. RT-PCR shows strong up-regulation of CD95L mRNA upon CD3 stimulation and significant inhibition of this induction by Dex cotreatment (Figure 3C, upper panel). However, inhibition of protein synthesis did not have any influence on the inhibitory effect of Dex (Figure 3C, lower panel). Activity of CHX was controlled by a cytotoxicity assay; CD3-induced apoptosis of day-5 primary T cells was blocked by CHX due to inhibition of CD95L protein synthesis (data not shown). These results clearly show that Dex-induced protein biosynthesis is not required to mediate the antiapoptotic effect of GCs on AICD. Therefore, participation of proteins such as GILZ and IκB can be excluded.
Interference with early TCR signaling is not involved in GR-mediated reduction of AICD
It has previously been suggested that high concentrations of Dex might interfere with early TCR signaling and tyrosine phosphorylation of components of the TCR/CD3 complex by disturbing recruitment of signaling components to lipid rafts.45 Figure 5A-B show that Dex inhibits activation-induced up-regulation of CD95L from early time points on, suggesting a direct mechanism on TCR signaling. To test this assumption, DC27.1 T cells were stimulated by anti-CD3 antibody for 5 hours, washed, and subsequently cultured for another 3 hours. For the last 3, 2, or 1 hour of this treatment Dex was added. CD95L was induced on the mRNA level upon TCR/CD3 stimulation for 5 hours, with 3 hours of additional cultivation in medium only. Importantly, addition of Dex still reduced CD95L mRNA levels after completion of TCR/CD3 stimulation (Figure 5C). This finding demonstrates that GCs regulate CD95L expression downstream of the formation of a TCR-signaling complex and thereby excludes a direct interference of Dex on early TCR signaling as the inhibitory mechanism.
GCs do not interfere with early TCR signaling, but inhibit AICD in vivo. DC27.1 T cells (A) and primary murine day 5 T cells (B) were (re-) stimulated by anti-CD3 antibody in the presence or absence of 10-7 M Dex for the indicated periods of time. CD95L protein expression on the cell surface was assayed by FACS analysis. (C) DC27.1 T cells were stimulated for 5 hours by anti-CD3 antibody, washed, and subsequently cultured for another 3 hours. For the last 1, 2, or 3 hours of this treatment 10-7 M Dex was added (lanes 2, 3, and 4, respectively). The mRNA expression of CD95L and β-actin was detected by RT-PCR. Of 3 experiments, 1 representative is shown. (D) C57BL/6 mice (8 weeks old) were pretreated for 24 hours with or without Dex-phosphate (300 μg/L) in the drinking water and subsequently injected intravenously with 5 μg anti-CD3 (+) or isotype control (-) antibody. Then, 20 hours later splenocytes were stained with anti-TCRβ and anti-B220 antibodies and analyzed by FACS (combined results of 5 independent experiments; each diamond represents one mouse; horizontal bars represent the mean; n = 8; t-value for CD3 vs CD3/Dex, P < .005).
GCs do not interfere with early TCR signaling, but inhibit AICD in vivo. DC27.1 T cells (A) and primary murine day 5 T cells (B) were (re-) stimulated by anti-CD3 antibody in the presence or absence of 10-7 M Dex for the indicated periods of time. CD95L protein expression on the cell surface was assayed by FACS analysis. (C) DC27.1 T cells were stimulated for 5 hours by anti-CD3 antibody, washed, and subsequently cultured for another 3 hours. For the last 1, 2, or 3 hours of this treatment 10-7 M Dex was added (lanes 2, 3, and 4, respectively). The mRNA expression of CD95L and β-actin was detected by RT-PCR. Of 3 experiments, 1 representative is shown. (D) C57BL/6 mice (8 weeks old) were pretreated for 24 hours with or without Dex-phosphate (300 μg/L) in the drinking water and subsequently injected intravenously with 5 μg anti-CD3 (+) or isotype control (-) antibody. Then, 20 hours later splenocytes were stained with anti-TCRβ and anti-B220 antibodies and analyzed by FACS (combined results of 5 independent experiments; each diamond represents one mouse; horizontal bars represent the mean; n = 8; t-value for CD3 vs CD3/Dex, P < .005).
GCs inhibit AICD in vivo
The data presented so far are based on experiments with cultured T cells. To demonstrate that GCs also have antiapoptotic function in vivo, we performed experiments in wild-type C57BL/6 mice pretreated with Dex phosphate. Subsequent T-cell apoptosis in vivo was induced by intravenous injection of anti-CD3 antibody.46 Splenic T cells were stained for TCRβ, distinguished from B cells by the B-cell marker B220, and analyzed by FACScan. The T-cell-to-B-cell ratio in the spleen of untreated mice is about 1. Upon anti-CD3 antibody injection, T cells undergo AICD,47-50 resulting in a reduced T-cell-to-B-cell ratio (Figure 5D). Pretreatment with subtoxic Dex concentrations within the drinking water, however, significantly (P < .005) reduced T-cell apoptosis in the treated mice (Figure 5D). The expression level of TCR was not changed by Dex treatment (data not shown). These data show that Dex is capable of reducing T-cell apoptosis in vivo, confirming our in vitro results.
The GR binds to a negative regulatory element (nGRE) in the CD95L promoter in vivo
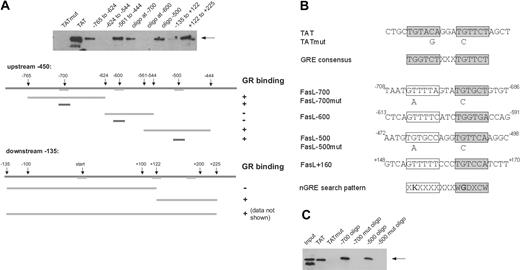
The results presented so far argue for a direct binding of the GR to the CD95L promoter, exerting its repressing effect by binding to an nGRE. Therefore, the promoter sequence of CD95L was searched in silico for potential nGREs. Since the DNA sequence of known nGREs is less conserved than the sequence pattern of positive GREs,51,52 we used a less stringent search pattern (XKXXXXXXXWGDXCW). In contrast to positive GREs that consist of 2 palindromic 6-bp halfsites separated by 3 nucleotides, nGREs lack the 5′ halfsite (only one base is weakly conserved), whereas the 3′ halfsite resembles a canonical binding site. For further analysis, the identified candidate DNA elements were investigated by an ABCD binding assay for their ability to bind GR in vitro (Figure 6A). Of 3 different sequences identified by this assay, 2 are located upstream (at -700 and -500) and 1 downstream (at +160) of the transcriptional start site (Figure 6A). To verify these putative nGRE elements, we created mutated sites as done before with the GRE for the TAT promoter52 (Figure 6B) and analyzed their GR binding properties (Figure 6C). There were 2 point mutations of conserved base pairs in each of the -700 and -500 DNA oligos that completely abolished GR DNA binding (Figure 6C). Finally, we applied a chromatin immunoprecipitation (ChIP) assay to assess the binding of GR, endogenously expressed in DC27.1 T cells, to the natural target promoter of the CD95L gene in vivo (Figure 7A-B). Regions of the CD95L promoter comprising those nGRE elements that were shown to be positive in the ABCD binding assay were analyzed by ChIP. Using this assay, we found that 2 of the CD95L promoter regions (namely those containing -700 and +160 nGRE sites) are capable of binding to the GR, whereas the -500 nGRE site is apparently not used by the GR in vivo (Figure 7A-B). Taken together, our data clearly show that the GR suppresses TCR-dependent up-regulation of CD95L in primary T cells via direct binding to several nGREs and thereby attenuates AICD in vivo.
Discussion
Due to their broad spectrum and strong immune suppressive effects, GCs are routinely used for treatment of acute and chronic inflammatory diseases. The anti-inflammatory activities of these drugs are due mainly to their capability to inhibit cytokine expression in lymphoid cells. The present study demonstrates a novel functional role for a direct DNA binding of the GR to the CD95L promoter, leading to a reduction of AICD in primary T lymphocytes.
A cross-talk between TCR and GR signaling has previously been suggested (mutual antagonism hypothesis) using mice overexpressing a GR antisense construct for the rat 3′ untranslated region (UTR) in thymocytes (TKOs).53 Ashwell et al postulate an antiapoptotic function of intrathymic GCs during negative thymocyte selection that interferes with TCR-induced deletion.54 Previous studies that used mice that carry a hypomorphic allele of GR (GRhypo) and express a truncated version of the GR protein with strongly reduced function36 demonstrated that the GR is not essential for thymocyte development and T-cell function.36-39 We have confirmed these results by using GRlckCre mice (A.B. et al, unpublished observations), in contrast to GRhypo and GR-TKO mice being a complete GR knock out in T cells (data not shown and Figure 4D-F).
The GR binds to an nGRE in the CD95L promoter. (A) The CD95L promoter was searched for potential GR binding sites by comparing the promoter sequence to the consensus of well-characterized nGREs (shaded lines). Candidate sites were analyzed in vitro for GR binding by ABCD assay (“Materials and methods”). Biotinylated DNA fragments of different sizes were incubated with Jurkat whole-cell extract as a source for GR. The GRE from the tyrosine aminotransferase promoter (TAT) served as a positive control; TAT oligo with a mutated GRE (TATmut), as negative control. (B) Overview of the binding elements used. Conserved halfsites are shaded gray; nonconserved halfsites are boxed. The nGRE search pattern is shown in International Union of Pure and Applied Chemistry (IUPAC) nomenclature. (C) Same as in panel A, but using DNA fragments identified as positive for GR binding containing mutations in the conserved base pairs of the putative nGREs. Only 50% of the TAT sample was loaded here compared with the other samples.
The GR binds to an nGRE in the CD95L promoter. (A) The CD95L promoter was searched for potential GR binding sites by comparing the promoter sequence to the consensus of well-characterized nGREs (shaded lines). Candidate sites were analyzed in vitro for GR binding by ABCD assay (“Materials and methods”). Biotinylated DNA fragments of different sizes were incubated with Jurkat whole-cell extract as a source for GR. The GRE from the tyrosine aminotransferase promoter (TAT) served as a positive control; TAT oligo with a mutated GRE (TATmut), as negative control. (B) Overview of the binding elements used. Conserved halfsites are shaded gray; nonconserved halfsites are boxed. The nGRE search pattern is shown in International Union of Pure and Applied Chemistry (IUPAC) nomenclature. (C) Same as in panel A, but using DNA fragments identified as positive for GR binding containing mutations in the conserved base pairs of the putative nGREs. Only 50% of the TAT sample was loaded here compared with the other samples.
AICD proceeds unchanged in GRlckCre mice lacking the GR in T lymphocytes, which shows that the downphase of a T-cell immune response does not depend on functional GR. However, we demonstrate here that the immune response cannot be modulated only by GC administration with respect to T-cell activation,15 but also during the downphase by regulating AICD. Our data show that inhibition of AICD in primary T cells by GCs is due to interference with CD95L expression, but not due to a direct influence on apoptosis signaling downstream of the CD95 death receptor.
GR binds to 2 nGREs in vivo. (A) DC27.1 T cells were either untreated (-) or treated (+) with 10-7 M Dex. Binding of GR to the -700, -500, and +160 CD95L promoter regions was assessed by chromatin immunoprecipitation (ChIP) assay. A known functional GRE-containing region of the mouse mammary tumor virus (MMTV) long terminal repeat (LTR) was used as a positive control for GR binding. As a negative control, the GR occupancy at the non-GRE-containing fragment of CD95L intron region approximately 7.5 kb downstream of the CD95L promoter was monitored. (B) Quantitative real-time PCR of DNA elements obtained from a ChIP experiment. Shown is percent of input precipitated of 1 of 3 experiments with similar results. Error bars represent mean ± SEM.
GR binds to 2 nGREs in vivo. (A) DC27.1 T cells were either untreated (-) or treated (+) with 10-7 M Dex. Binding of GR to the -700, -500, and +160 CD95L promoter regions was assessed by chromatin immunoprecipitation (ChIP) assay. A known functional GRE-containing region of the mouse mammary tumor virus (MMTV) long terminal repeat (LTR) was used as a positive control for GR binding. As a negative control, the GR occupancy at the non-GRE-containing fragment of CD95L intron region approximately 7.5 kb downstream of the CD95L promoter was monitored. (B) Quantitative real-time PCR of DNA elements obtained from a ChIP experiment. Shown is percent of input precipitated of 1 of 3 experiments with similar results. Error bars represent mean ± SEM.
As a ligand-inducible transcription factor, the GR can directly interfere with other transcription factors,23 as documented for repressive effects on AP-1 and NF-κB activities.13-15 It as been shown that GC-mediated regulation of cytokine expression is mediated by GR monomers, which bind and repress AP-1 and NF-κB activities.15,17 Although NF-κB and AP-1 are shown to be essential for activation-induced CD95L expression in T cells,9 we cannot observe negative regulation of CD95L expression in GRdim mice.25 Thus, our results rule out that transrepression of transcription factors such as NF-κB or AP-1 via direct interaction with a GR monomer is involved in the antiapoptotic function of GCs in primary peripheral T lymphocytes.
GC-induced expression of proteins that regulate CD95L expression (eg, GILZ,42-44 IκB,40,41 and IL-7 receptor [IL-7R]55 ) has been suggested to regulate T-cell apoptosis. Yet inhibition of protein biosynthesis by cycloheximide does not effect Dex-mediated modulation of CD95L expression. Thus, indirect effects by GC-induced expression of other regulatory molecules do not contribute to the antiapoptotic effect of Dex on AICD.
As expected, the identified nGREs in the CD95L promoter do not match the consensus sequence of positive GREs. In contrast to numerous genes known to be down-regulated by mechanisms involving transrepression, only a few nGRE-containing promoters have been characterized in detail.56-58 nGREs have a lower binding affinity toward GR in vitro compared with GREs.59 As GREs and nGREs have different structures, the mode of binding may also be different. The presence of several nGREs within the CD95L promoter suggests a mechanism of cooperative binding where GR dimers bind to several adjacent elements, stabilize each other, and enable repression of CD95L gene transcription. Cooperative binding has been shown for the phosphoenolpyruvate carboxykinase (PEPCK) promoter in the context of gene activation.60 Obviously, the ability to dimerize is required for successful repression, as shown for the POMC gene,25 explaining the inability of Dex to modulate CD95L expression in GRdim mice.
Different mouse models have confirmed the involvement of the CD95 signaling pathway in AICD in vivo.47-50 Injection of a depleting anti-CD3 antibody (145-2C11) at a low dose serves as an in vivo model for CD95-dependent AICD.61 We used this approach to verify the in vitro observed antiapoptotic effect of Dex on T-cell apoptosis in vivo. T-cell deletion in vivo was measured by analyzing T-cell-to-B-cell ratio, as apoptotic cells in vivo are hardly detectable due to rapid uptake by surrounding phagocytic cells.62 Most importantly, intravenous injection of anti-CD3 antibody reduced the number of T cells within the spleen by inducing apoptosis, while cotreatment of mice with Dex in the drinking water reduced in vivo T-cell depletion. Expression of the TCR itself was not altered by GC treatment. Although T cells become more susceptible toward GC-induced apoptosis under stress conditions, Dex concentrations used in this study do not induce peripheral T-cell apoptosis in vivo.
Taken together, our results support the hypothesis that negative regulation of CD95L expression in T cells might contribute to the anti-inflammatory function of GCs. Cells dying by apoptosis in vivo are rapidly cleared by macrophages, whereas the presence of apoptotic cells during monocyte activation modulates cytokine expression, hence creating an anti-inflammatory milieu.63 However, when massive cell death overwhelms macrophage clearance as found in strong inflammation or viral infections,64 apoptotic cells may progress to secondary necrosis.65,66 Necrosis may then trigger inflammation in vivo.67 Therefore, tissue damage induced by CD95L-expressing effector T lymphocytes, inducing massive apoptosis in the surrounding tissue, might promote inflammation. In addition, the CD95L has been reported to induce the processing and release of IL-1β, leading to neutrophil infiltration into the inflamed organs.68,69 Moreover, engagement of CD95 on dendritic cells may induce the secretion of proinflammatory cytokines.70 Recently, CD95L has been confirmed to serve as an inflammatory factor under certain circumstances.71,72 The reduction in CD95L expression in activated T cells, as shown in this study, might therefore make an important contribution to the anti-inflammatory effect of glucocorticoid drugs during treatment of inflammatory diseases. This is in line with previous observations that CD95L contributes to the rejection of transplanted tissue73 and T-cell-mediated inflammatory bowel diseases, such as celiac and graft-versus-host disease. In fact, Dex was shown to reduce tissue damage in respective mouse models,74 and reduce CD95L expression, induced by treatment with chemotherapeutic drugs such as cisplatin.75 In addition, increased cytokine production due to T-cell activation leads to stimulation of the hypthalamo-pituitary-adrenal axis (HPA), resulting in increased GC serum levels.76 We observed strongly increased GC serum levels after 3 hours of TCR stimulation in vivo (data not shown). Although these hormones rescue effector T cells from AICD, the surviving T cells show an altered gene expression pattern, inhibiting their effector function.15 Future studies using specifically engineered cells with mutated nGREs in the CD95L promoter will finally have to formally demonstrate that the inhibition of AICD is dependent on GR binding in vivo.
In summary, direct DNA-dependent repression of CD95L expression in peripheral effector T cells by the GR in vivo can be considered a mechanism contributing to the anti-inflammatory effect of GCs. This study increases our knowledge of the role of CD95-mediated apoptosis during steroid treatment of inflammatory diseases and might enable the development of novel glucocorticoid drugs with reduced metabolic side effects in the future.
Prepublished online as Blood First Edition Paper, March 31, 2005; DOI 10.1182/blood-2004-11-4390.
Supported by Deutsche Forschungsgemeinschaft grant SFB405 and the Sander Stiftung.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors like to thank Pola Linzmayer, Simone Stößer, Sascha Wey, Myriam Grüner, Christine Stumpf, Kathrin Kappes, Marco Giaisi, and Wolfgang Müller for excellent technical assistance. We are grateful to Heidi Sauter for expert secretarial assistance and Dr Henning Schulze-Bergkamen for taking blood samples. We thank Dr Inna Lavrik, Dr Rüdiger Arnold, and Kerstin Florin for critically reading the manuscript and Dr Min Li-Weber for helpful discussions.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal