Abstract
B lymphocytes respond to bacterial lipopolysaccharide (LPS) through Toll-like receptor 4 (TLR4) and CD180 (previously called RP105). We show here that the responses of B lymphocytes to LPS require the function of the Vav family of guanine nucleotide exchange factors. Vav1-mutant mice generate defective humoral immunoglobulin G (IgG) responses following administration of low doses of LPS but respond normally to higher doses, while mice lacking both Vav1 and Vav2 manifest defective responses even after a high dose of LPS. Vav1/2-mutant B cells fail to divide extensively in vitro in response to LPS or CD180, while deficiency of Vav1 alone impairs CD180-but not LPS-driven proliferation. Likewise, activation of Akt (a PI3K [phosphatidylinositol 3-kinase] target) and phosphorylation of IκBα in response to CD180 or LPS required Vav1 and Vav2, while Vav1 deficiency led to defective responses to CD180. In addition, activation of ERK (extracellular signal regulated kinase) required Vav1 and Vav2 in response to CD180 but was Vav1 and vav2 independent in response to LPS. Induction of CD86 and CD25 by anti-CD180 also required Vav function, as did the induction of the antiapoptotic protein Bcl-xL (B-cell leukemia XL). These data provide evidence for the function for the Vav proteins in regulating the responses of B cells to LPS. (Blood. 2005;106:635-640)
Introduction
Bacterial lipopolysaccharide (LPS) is a potent B-cell mitogen both in vivo1 and in vitro.2 Activation of B cells by LPS triggers immunoglobulin (Ig) secretion and Ig class switching.3 LPS also promotes the ability of B cells to function as antigen-presenting cells by increasing expression of major histocompatibility complex type II (MHC-II) and costimulatory molecules such as CD86.4,5 Collectively, these responses of B cells contribute to the neutralization of Gram-negative bacteria.
B cells express 2 receptors that function as recognition and signal-transducing receptors for LPS. Toll-like receptor 4 (TLR4) is a leucine-rich repeat protein with a large cytoplasmic domain that contains a signal transducing Toll/IL-1 (interleukin 1) receptor (TIR) homology domain.6 CD180, previously called RP105, similarly contains a leucine-rich extracellular domain but bears an 11-amino acid cytoplasmic domain with no homology to TLR4 or other known proteins.7 The extracellular domains of TLR4 and CD180 associate with small molecules called MD2 and MD1, respectively. These molecules contribute to recognition of LPS as well as the surface expression and intracellular distribution of TLR4 and CD180.8,9 Although B cells express relatively little TLR4 when compared with macrophages, analysis of mutant mice demonstrate TLR4 is essential for B-cell responses to LPS. Engineered mutations of CD180 and MD1 have revealed roles for each of these in LPS-mediated activation of B cells and in LPS-induced antibody responses.9,10 While mutation of TLR4 yields a more severe defect in humoral LPS responses than that of CD180 or MD1, the genetic experiments indicate a nonredundant requirement for both classes of LPS receptor for the optimal responses of B cells.
TLR4 is ubiquitously expressed, and the signal transduction pathways it activates have been well studied. The cytoplasmic domain of TLR4 recruits adapter proteins MyD88 (myeloid differentiation factor 88), TIRAP (TIR-containing adaptor protein), and TRIF (TIR-containing adaptor protein inducing interferon β), which, in turn, lead to the activation of ERK (extracellular receptor-activated kinase), JNK (c-JUN n-terminal kinase), and p38 MAP (mitogen-activated protein) kinases and subsequently to the activation of transcription factors, including NF-κB (nuclear factor κB) and IRF3 (interferon response factor 3) (reviewed by Sabroe et al11 ). By contrast, expression of CD180 is restricted to mature B lymphocytes. However, the mechanisms by which CD180 signals are less well studied. CD180 crosslinking by antibody leads to activation of ERK, JNK, and p38.12,13 B cells deficient in MyD88 proliferate normally following CD180 crosslinking,14 while B cells deficient in PKCβ (protein kinase C β) or from xid (X-linked immunodeficient) mice lacking normal BTK (Bruton tyrosine kinase) function, respond poorly to CD180 crosslinking.13 Recently, it has been reported that the B-cell coreceptor CD19 is required for optimal proliferative responses to CD180 ligation.14 Furthermore, it was suggested that Vav1 may participate in CD180 signaling by virtue of its association with CD19.14
The Vav family of guanine nucleotide exchange factors (GEFs) consists of 3 members, each of which is phosphorylated in response to BCR (B-cell receptor) crosslinking.15 There is genetic evidencethat all 3 Vav proteins contribute to signaling by the BCR.16 However, there is significant redundancy between the family members. We and others have shown that B cells from mice deficient in Vav proteins are defective in both thymus-dependent and type II-thymus-independent responses.16-18 However, the responses of Vav-deficient mice to the type I-thymus-independent antigen LPS has not been investigated.
In this study we show Vav proteins are required for normal class-switched antibody production following immunization with a hapten-LPS conjugate. In the absence of normal Vav function, LPS- and CD180-stimulated signal transduction, gene expression, cell survival, and proliferation are defective. The results indicate that Vav protein function is necessary for the response of B cells to LPS.
Materials and methods
Mice and immunization
Vav1-/-, Vav2-/-, and Vav1/2-/- mice have been described previously.17 For this study, all mice were backcrossed to the B10.BR background for 5 generations, as CD180 responses are defective in mice on the 129/Sv background.19 Naive mice where injected intravenously via the tail vein with 20 μg or 3 μg DNP (dinitrophenyl (0.3)-LPS (Biosearch Technologies, Novato, CA) in 200 μL phosphate-buffered saline (PBS). Sera were collected on days 0, 7, and 14 for analysis for DNP-specific antibodies by enzyme-linked immunosorbent assay (ELISA) as previously described.17
B-cell purification and antibody secretion
B cells were purified from spleens by negative selection to purities between 87% and 97% using previously described methods.17 Purified B cells/well (1.5 × 105) were cultured with 20 μg/mL LPS (strain 0111:B4; Sigma-Aldrich, Poole, United Kingdom) in the presence of 100 U/mL IL-4 (R&D Systems, Oxford, United Kingdom) and 1.2 ng/mL IL-5 (Sigma) or without LPS for 5 to 6 days in 96-well round-bottom plates. Supernatants were diluted 1:10 for IgG1 and IgG3 and 1:1000 for IgM and analyzed by ELISA using purified rat anti-mouse IgG1, IgG3, and IgM for capture and 2 μg/mL biotinylated rat anti-mouse IgG1, IgG3, and IgM (Becton Dickinson, Oxford, United Kingdom) for detection.
Cell cycle, survival, and proliferation assay
For cell-cycle analysis 2 × 105 purified B cells/well were stimulated either with 5 μg/mL anti-CD180 (Becton Dickinson), 1 μg/mL LPS, or cultured without stimulus for 24 and 48 hours in 96-well round-bottom plates. Cells were harvested, washed, and permeabilized in 1 mL ice-cold 70% EtOH in PBS by vortexing for 30 seconds and incubated on ice for 40 minutes. Cells were washed in PBS and 100 μg/mL RNaseA was added in 50 μL, incubated at room temperature (RT) for 10 minutes, and then stained in 50 μg/mL PI (propidium iodide) in PBS. Samples were analyzed by flow cytometry for linear PI fluorescence intensity gating on single cells. For analysis of proliferation 1 × 107 purified B cells/mL were stained in 1 μM CFDA-SE (5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester; Vybrant CFDA-SE cell tracer kit; Molecular Probes, Eugene, OR) in PBS at RT for 10 minutes. Cells were washed in RPMI containing 10% FCS (fetal calf serum), seeded into 96-well round-bottom plates at 2 × 105 cells/well, and incubated at 37°C for 30 minutes. LPS or anti-CD180 was added to a final concentration of 1 μg/mL and 5 μg/mL, respectively, and cells were cultured for 48 hours and 72 hours. Cells were harvested and stained with 10 nM TO-PRO-3 immediately before acquisition to allow for the identification of dead cells.
Flow cytometry of splenocytes
For detection of surface activation markers, cultured purified B cells were stained with anti-CD25-PE (phycoerythrin) or anti-CD86-Biotin. Biotinylated antibodies were detected using streptavidin-QuantumRed (Sigma). For intracellular Bcl-xL (B-cell leukemia XL), cultured B cells were fixed with Fix/Perm solution (Becton Dickinson) and permeabilized in 0.03% Saponin in PBS/0.5% BSA (bovine serum albumin) on ice. They were then stained with anti-Bcl-xL-PE or isotype control (Southern Biotechnology, Birmingham, AL) and analyzed using fluorescence-activated cell sorting (FACS) Calibur flow cytometer with Cellquest software for acquisition and Flojo software (Tree Star, Ashland, OR) for analysis.
Defective TI-1 responses. Eight-week-old WT (□), Vav1-/- (▦), Vav2-/- ( ), and Vav1/2-/- (▪) mice were injected intravenously with 20 μg (A) or 3 μg (B) DNP-LPS. Sera were collected at the times shown and analyzed by ELISA for DNP-specific antibodies of the isotypes indicated. *P < .05, **P < .005 by analysis of variance (ANOVA); n = 5. Bar graphs represent the mean of the optical density (OD) values for 5 mice per group, and error bars correspond to the standard error of the mean. (C) Purified B cells of WT and Vav-deficient mice (genotypes as described above) were cultured for 5 days in the presence of 20 μg/mL LPS, and secreted antibodies were analyzed by ELISA. Error bars indicate standard deviation of 6 wells; *P < .05, **P < .005 by ANOVA.
), and Vav1/2-/- (▪) mice were injected intravenously with 20 μg (A) or 3 μg (B) DNP-LPS. Sera were collected at the times shown and analyzed by ELISA for DNP-specific antibodies of the isotypes indicated. *P < .05, **P < .005 by analysis of variance (ANOVA); n = 5. Bar graphs represent the mean of the optical density (OD) values for 5 mice per group, and error bars correspond to the standard error of the mean. (C) Purified B cells of WT and Vav-deficient mice (genotypes as described above) were cultured for 5 days in the presence of 20 μg/mL LPS, and secreted antibodies were analyzed by ELISA. Error bars indicate standard deviation of 6 wells; *P < .05, **P < .005 by ANOVA.
Defective TI-1 responses. Eight-week-old WT (□), Vav1-/- (▦), Vav2-/- ( ), and Vav1/2-/- (▪) mice were injected intravenously with 20 μg (A) or 3 μg (B) DNP-LPS. Sera were collected at the times shown and analyzed by ELISA for DNP-specific antibodies of the isotypes indicated. *P < .05, **P < .005 by analysis of variance (ANOVA); n = 5. Bar graphs represent the mean of the optical density (OD) values for 5 mice per group, and error bars correspond to the standard error of the mean. (C) Purified B cells of WT and Vav-deficient mice (genotypes as described above) were cultured for 5 days in the presence of 20 μg/mL LPS, and secreted antibodies were analyzed by ELISA. Error bars indicate standard deviation of 6 wells; *P < .05, **P < .005 by ANOVA.
), and Vav1/2-/- (▪) mice were injected intravenously with 20 μg (A) or 3 μg (B) DNP-LPS. Sera were collected at the times shown and analyzed by ELISA for DNP-specific antibodies of the isotypes indicated. *P < .05, **P < .005 by analysis of variance (ANOVA); n = 5. Bar graphs represent the mean of the optical density (OD) values for 5 mice per group, and error bars correspond to the standard error of the mean. (C) Purified B cells of WT and Vav-deficient mice (genotypes as described above) were cultured for 5 days in the presence of 20 μg/mL LPS, and secreted antibodies were analyzed by ELISA. Error bars indicate standard deviation of 6 wells; *P < .05, **P < .005 by ANOVA.
Cell sorting and real-time PCR
Splenocytes were stained with anti-CD23 and anti-CD21 antibodies. Follicular and marginal zone B cells were identified as CD21+ and CD23+ or CD21high and CD23low, respectively, and sorted on an Aria cell sorter (Becton Dickinson). Reanalysis of sorted cells revealed greater than 97% purity. For gene expression analysis RNA was extracted using TRIzol reagent (Invitrogen, Paisley, United Kingdom), and cDNA was prepared using high-capacity cDNA Archive kit (Applied Biosystems, Oxford, United Kingdom) according to the manufacturer's instructions. Real-time polymerase chain reactions (PCRs) used Taq-Man Universal Master Mix (Applied Biosystems) and an ABI Prism 7700 Sequence Detection System. TLR4 RNA concentrations were calculated relative to HPRT (hypoxanthine phosphoribosyltransferase) expression. Primers and FAM (6-carboxyfluorsecein)-labeled probes for TLR4 and HPRT were obtained from Applied Biosystems (assays on demand).
Western blot analysis of phospho-proteins and JNK assay
For Western blots 107 purified B cells/condition were stimulated in RPMI with 10% FCS. Cells were spun down, lysed by adding 50 mM Tris (tris(hydroxymethyl)aminomethane) pH 7.4, 80 mM KCl, 10 mM EDTA (ethylenediaminetetraacetic acid), 1% NP-40 (Nonidet P-40, [Octylphenoxy] polyethoxyethanol), and phosphatase and protease inhibitors (1 mM Na3VO4, 5 mM NaF, 10 μM leupeptin, 2 μg/mL antipain, 6 μg/mL chymostatin, 1 μM pepstatin A, 1 μg/mL AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride]); and incubated for 5 minutes on ice. Detergent-insoluble fractions were removed by centrifugation, and supernatants were analyzed by Western blot. For JNK assay, cells were lysed for 20 minutes on ice in 150 μL kinase assay lysis buffer (25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 7.6, 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, phosphatase and protease inhibitors), diluted with 450 μL ice-cold dilution buffer (25 mM HEPES pH 7.6, 2.5 mM MgCl2, 0.05 mM EDTA, 0.025% Triton X-100, phosphatase and protease inhibitors) per sample and incubated with 15 μL GST (glutathione S-transferase)-Jun(aa1-91) conjugated to glutathione beads for 1 hour. Beads were washed with ice-cold wash buffer (20 mM HEPES pH 7.6, 50 mM NaCl, 2.5 mM MgCl2, 0.1 mM EDTA, 0.05% Triton X-100, phosphatase and protease inhibitors) and incubated for 30 minutes at 30°C in kinase reaction buffer (20 mM HEPES pH 7.6, 20 mM MgCl2, 2 μM DTT (dithiothreitol), 20 μM β-glycerophosphate, phosphatase inhibitors, 25 μM ATP [adenosine triphosphate]); beads were spun down and analyzed for phosphorylated c-Jun by Western blot. Antibodies used were phospho-serine-473 Akt (a PI3K [phosphatidylinositol 3-kinase] target), phospho-IκBα, phospho-Jun, and phospho-ERK (rabbit; Cell Signaling Technologies, Hitchin, United Kingdom), IκBα and ERK (rabbit; Santa Cruz Biotechnolgy, Santa Cruz, CA), followed by horseradish peroxidase-conjugated goat antirabbit (Dako, Ely, United Kingdom).
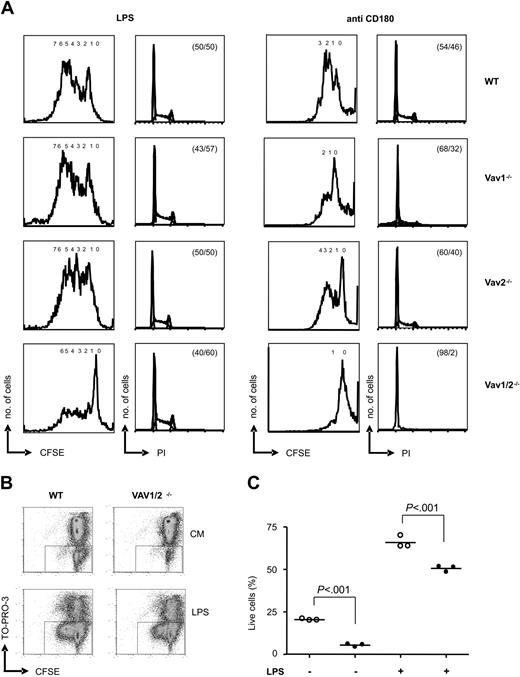
Requirement for Vav proteins in cell-cycle progression. (A) B cells of the indicated genotypes were stained with CFDA-SE and cultured in the presence of 1 μg/mL LPS (left) or 5 μg/mL anti-CD180 (right). The first and third panels show analyses of cells gated for lymphocytes by forward and side scatter of CFDA-SE fluorescence after 72 hours of culture; numbers of divisions are indicated. The second and fourth panels show cell-cycle analyses of PI staining after 48 hours of culture; percentage of cells in G1 and G2/S/M phases are given. CFSE indicates carboxyfluorescein diacetate succinimidyl ester. (B) B cells from WT or Vav1/2-/- mice were loaded with CFDA-SE and cultured with or without 1 μg/mL LPS. After 48 hours, cells were harvested, stained with TO-PRO-3, and analyzed by FACS. Results from a representative mouse of each genotype are displayed. The boxes indicate live cells identified as TO-PRO-3 negatives. CM indicates culture medium. (C) The graph shows the percentage of live cells from 3 WT (○) or 3 Vav1/2-/- mice (•) after culture with or without LPS for 48 hours. Statistical significance was determined using the Student t test. Error bars represent the mean of the 3 values.
Requirement for Vav proteins in cell-cycle progression. (A) B cells of the indicated genotypes were stained with CFDA-SE and cultured in the presence of 1 μg/mL LPS (left) or 5 μg/mL anti-CD180 (right). The first and third panels show analyses of cells gated for lymphocytes by forward and side scatter of CFDA-SE fluorescence after 72 hours of culture; numbers of divisions are indicated. The second and fourth panels show cell-cycle analyses of PI staining after 48 hours of culture; percentage of cells in G1 and G2/S/M phases are given. CFSE indicates carboxyfluorescein diacetate succinimidyl ester. (B) B cells from WT or Vav1/2-/- mice were loaded with CFDA-SE and cultured with or without 1 μg/mL LPS. After 48 hours, cells were harvested, stained with TO-PRO-3, and analyzed by FACS. Results from a representative mouse of each genotype are displayed. The boxes indicate live cells identified as TO-PRO-3 negatives. CM indicates culture medium. (C) The graph shows the percentage of live cells from 3 WT (○) or 3 Vav1/2-/- mice (•) after culture with or without LPS for 48 hours. Statistical significance was determined using the Student t test. Error bars represent the mean of the 3 values.
Results
LPS-stimulated IgG production in mice requires Vav function
To further investigate the involvement of Vav proteins in B-cell responses we analyzed the thymus-independent type I (TI-I) antibody responses of mice with single or combined mutations in Vav1 and Vav2. To this end, groups of wild-type (WT), Vav1-/-, Vav2-/-, and Vav1/2-/- mice were injected with 2 different doses of DNP-LPS, and sera were analyzed for DNP-specific antibodies of the IgM, IgG2a, IgG2b, and IgG3 subclasses. At the high dose of DNP-LPS Vav1/2-/- mice produced levels of antigen-specific IgM that were similar to WT at day 7 but significantly higher than WT on day 14 (Figure 1A).
By contrast, there were significantly lower levels of antigen-specific IgG2a, IgG2b, and IgG3 in sera collected on days 7 and 14 from Vav1/2-/- mice (Figure 1A). When injected with a lower dose of DNP-LPS, Vav1/2-/- mice produced normal levels of antigen-specific IgM and reduced levels of antigen-specific IgG2a, IgG2b, and IgG3 (Figure 1B). Additionally, Vav1-/- mice, which responded in a similar manner to WT mice when administered the high dose of DNP-LPS, produced normal levels of DNP-specific IgM but significantly decreased levels of antigen-specific IgG2b and IgG3 after challenge with the low dose of antigen (Figure 1B). Antigen-specific antibody levels in immunized Vav2-/- mice resembled those of WT mice at both doses of DNP-LPS. These experiments demonstrate a clear defect in the class-switched TI-I response of mice lacking Vav1 or both Vav1 and Vav2. This defect is manifested in Vav1/2-/- mice at both high and low doses of antigen and in Vav1-/- mice at low doses of antigen. To investigate whether this defect was due to decreased responsiveness of Vav-deficient B cells to LPS, we purified splenic B cells from the 4 genotypes and tested their antibody secretion in response to LPS in vitro. Purified splenic B cells (purities of > 90%) were incubated in the presence of LPS, and culture supernatants were analyzed for IgM, IgG1, and IgG3. B cells lacking Vav1 or Vav2 produced lower levels of IgM than WT, and this was further reduced in B cells lacking both Vav1 and Vav2 (Figure 1C). The reduced IgM secretion following addition of LPS to the in vitro cultures of B cells contrasts with our in vivo data that showed antigen-specific IgM production was unimpaired in Vav1-/-, Vav2-/-, and Vav1/2-/- mice. This may reflect the involvement of other cell types, absent from the in vitro assay, for the normal IgM responses of B cells lacking Vav proteins. Following in vitro LPS stimulation Vav1-/- and Vav1/2-/- B cells generated reduced levels of IgG1 and IgG3, while Vav2-/- B cells yielded IgG levels similar to WT B cells (Figure 1C). Collectively, these results demonstrate that Vav proteins are necessary for optimal production of class-switched antibodies in response to LPS. The data suggest that there may be some redundancy between Vav1 and Vav2 when LPS concentrations are high but that Vav1 is required when LPS concentrations are low.
Proliferation in response to LPS and anti-CD180 requires Vav function
We next tested the ability of purified B cells to progress through the cell cycle and to proliferate in response to LPS and CD180 crosslinking. In response to LPS WT, Vav1-/-, Vav2-/-, and Vav1/2-/- B cells all progressed to S/G2 (Figure 2A). In response to anti-CD180 there was reduced DNA synthesis in Vav1-/- B cells. However, there was virtually no detectable DNA replication in Vav1/2-/- B cells (Figure 2A). By staining cells with CFDA-SE we measured the proportion of cells that divided and how many divisions individual cells underwent. In response to CD180 stimulation, proliferation of Vav1-/- B cells was reduced, and there was virtually no proliferation of Vav1/2-/- B cells. Only a very small proportion of WT, Vav1-/-, and Vav2-/- B cells failed to proliferate in response to LPS (Figure 2A). By contrast, a large proportion of Vav1/2-/- B cells treated with LPS did not divide at all. However, a minority population of Vav1/2-/- B cells underwent a similar number of cell divisions as did the majority of WT B cells. These results are consistent with the reduced thymidine incorporation in Vav1/2-/- B cells in response to LPS18 (E.V., unpublished data, 2003). Vav1/2-/- B cells survived less well when compared with WT after culture in media alone or in media with LPS (Figure 2B-C). However, the failure of Vav1/2-/- B cells to proliferate was not only due to cell death, as LPS still rescued a large fraction of Vav1/2-/- B cells from apoptosis (Figure 2C). Even though Vav1/2-/- B cells are able to progress through the cell cycle in response to LPS, they do not divide as efficiently. This might be the basis for the reduced antibody secretion since proliferation has been implicated as a prerequisite of switched antibody production.20 Our results indicate that Vav proteins are required for both the proliferative and survival responses of B cells following exposure to LPS.
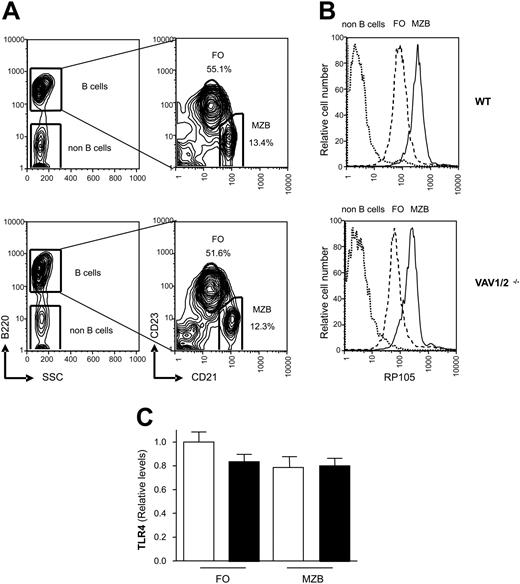
Expression of CD180 and TLR4 in WT and Vav1/2-/- mice. (A) Five percent probability contour plots showing CD21 and CD23 surface staining of B220+ gated splenocytes from WT and Vav1/2-/- mice. MZ B cells fall within the CD21high CD23low gate, and the percentage of MZ and FO B cells are indicated. SSC indicates side scatter. (B) Expression of CD180 on FO and MZ B cells from WT and Vav1/2-/- mice using the gating from panel A. (C) Marginal zone and follicular B cells from WT or Vav1/2-/- mice were FACS sorted according to CD21 and CD23 levels as described in panel A. Expression of TLR4 was measured by real-time PCR on cDNA from the indicated populations; WT mice (□) and Vav1/2-/- mice (▪). No significant differences were observed between the groups. Error bars indicate standard deviation.
Expression of CD180 and TLR4 in WT and Vav1/2-/- mice. (A) Five percent probability contour plots showing CD21 and CD23 surface staining of B220+ gated splenocytes from WT and Vav1/2-/- mice. MZ B cells fall within the CD21high CD23low gate, and the percentage of MZ and FO B cells are indicated. SSC indicates side scatter. (B) Expression of CD180 on FO and MZ B cells from WT and Vav1/2-/- mice using the gating from panel A. (C) Marginal zone and follicular B cells from WT or Vav1/2-/- mice were FACS sorted according to CD21 and CD23 levels as described in panel A. Expression of TLR4 was measured by real-time PCR on cDNA from the indicated populations; WT mice (□) and Vav1/2-/- mice (▪). No significant differences were observed between the groups. Error bars indicate standard deviation.
CD180 and TLR4 expression by WT and Vav1/2-/- B cells
To confirm that the failure to respond to anti-CD180 or LPS treatment was not due to reduced CD180 or TLR4 expression we analyzed CD180 levels by flow cytometry and TLR4 levels by real-time PCR. Marginal zone (MZ) and follicular (FO) B cells can be distinguished through differential expression of CD21 and CD23, and the spleens of WT and Vav1/2-/- mice contain similar percentages of these 2 subsets (Figure 3A). The expression of CD180 is about 4.5 times higher on MZ when compared with FO B cells of WT or Vav1/2-/- mice, but CD180 expression does not differ between WT and Vav1/2-/- B cells (Figure 3A). To examine the expression of TLR4 we used real-time PCR (Figure 3C). TLR4 mRNA was expressed equivalently between FO and MZ B-cell subsets from WT and Vav1/2-/- mice. We conclude that the defects Vav1/2-/- B cells display in response to CD180 or LPS are unlikely to result from reduced CD180 or TLR4 expression. We further conclude that the failure of Vav1/2-/- mice to respond to LPS is not a consequence of the absence of MZ B cells which have been shown to be more responsive to LPS than FO B cells.21 Moreover, the elevated expression of CD180 on MZ B cells may contribute to the heightened LPS responsiveness of this population.
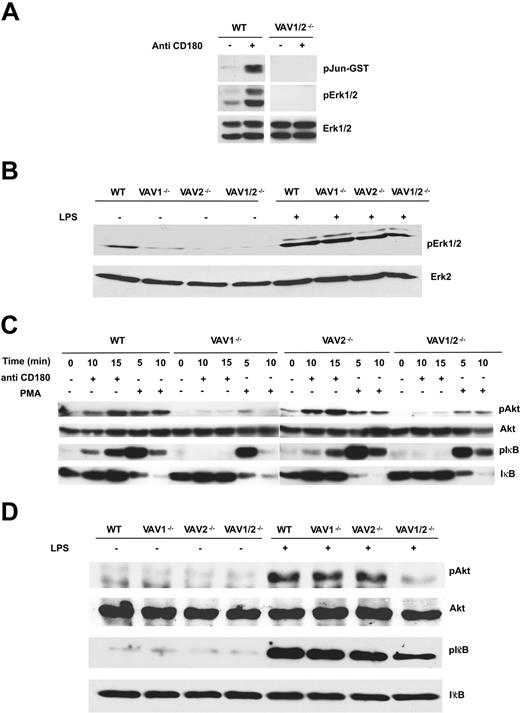
Vav-deficient mice show defects in MAP kinase, Akt, and IκB activation in response to LPS or anti-CD180 stimulation. B cells were stimulated with (A) anti-CD180 (5 μg/mL for 15 minutes), then analyzed for activation of JNK and ERK. (Top) JNK activity assessed by measuring c-Jun phosphorylation. (Middle) ERK activity assessed by blotting with anti-phospho ERK. (Bottom) Total ERK levels assessed by blotting with anti-ERK1 and -ERK2 antibodies. (B) LPS (10 μg/mL for 20 minutes) was then analyzed for activation of ERK activation using anti-phospho ERK. In this experiment anti-ERK-2 was used as the loading control. (C) 5 μg/mL anti-CD180 or 50 ng/mL PMA (phorbol 12-myristate-13-acetate) for the indicated times or (D) 10 μg/mL LPS for 20 minutes. Akt and IκBα phosphorylation were assessed by Western blot using phosphospecific antibodies. Subsequently, blots were stripped and reprobed with Akt or IκBα antibodies, respectively, to confirm equivalent protein loading.
Vav-deficient mice show defects in MAP kinase, Akt, and IκB activation in response to LPS or anti-CD180 stimulation. B cells were stimulated with (A) anti-CD180 (5 μg/mL for 15 minutes), then analyzed for activation of JNK and ERK. (Top) JNK activity assessed by measuring c-Jun phosphorylation. (Middle) ERK activity assessed by blotting with anti-phospho ERK. (Bottom) Total ERK levels assessed by blotting with anti-ERK1 and -ERK2 antibodies. (B) LPS (10 μg/mL for 20 minutes) was then analyzed for activation of ERK activation using anti-phospho ERK. In this experiment anti-ERK-2 was used as the loading control. (C) 5 μg/mL anti-CD180 or 50 ng/mL PMA (phorbol 12-myristate-13-acetate) for the indicated times or (D) 10 μg/mL LPS for 20 minutes. Akt and IκBα phosphorylation were assessed by Western blot using phosphospecific antibodies. Subsequently, blots were stripped and reprobed with Akt or IκBα antibodies, respectively, to confirm equivalent protein loading.
CD180 and LPS signal transduction requires Vav proteins
We next sought to determine the signaling pathways that Vav proteins were mediating for LPS and anti-CD180. CD180 stimulation induced activation of ERK and JNK that was defective in Vav1/2-/- B cells (Figure 4A). By contrast, ERK activation by LPS was readily apparent in B cells lacking both Vav1 and Vav2 (Figure 4B). JNK activation was not consistently observed in WT B cells activated by LPS (data not shown); thus, we have not been able to establish a requirement for Vav proteins in LPS-mediated JNK activation. As Vav proteins have been shown to regulate PI3K activation in response to diverse stimuli in several cell types,15 we used Akt phosphorylation on serine 473 as a surrogate for PI3K activation. The phosphorylation of Akt in response to CD180 stimulation was much reduced in the absence of Vav1 or Vav1/2 (Figure 4C). Akt phosphorylation in response to LPS was unaffected by the lack of Vav1 but was reduced in Vav1/2-/- B cells (Figure 4D). These data indicate Vav proteins contribute to PI3K activation by LPS in B cells. As Vav proteins have previously been shown to be dispensable for PI3K activation following surface immunoglobulin crosslinking,22 the data presented here further indicate that the requirement for Vav proteins in PI3K activation is stimulus specific. Activation of NF-κB by LPS and CD180 is necessary for the B-cell response to these mitogens.23,24 We measured phosphorylation of IκBα as a marker for the induction of this class of transcription factor. Following CD180 stimulation phosphorylation of IκBα was defective in the absence of Vav1 alone or both Vav1 and Vav2 (Figure 4C). By contrast, LPS-stimulated IκBα phosphorylation was unaffected by the lack of Vav1 (Figure 4D). Instead, IκBα phosphorylation induced by LPS was reduced in B cells lacking Vav1 and Vav2 (Figure 4D). PMA-stimulated IκBα phosphorylation was normal in B cells of all genotypes, suggesting the IKK (IκB kinase) complex was functional (Figure 4C). Taken together these results suggest the signaling pathways activated by LPS and CD180 require Vav function. CD180 requires Vav1, while LPS activation of some signaling pathways in B cells can bypass the loss of Vav1 presumably by using Vav2.
Vav function is required for activation of gene expression
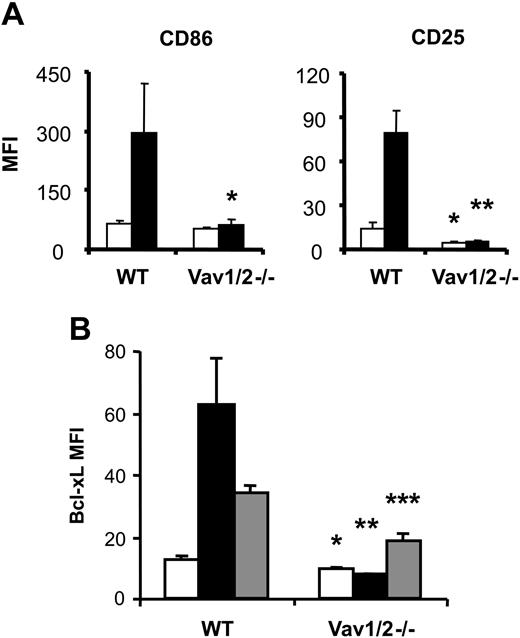
Increased expression of CD25 and CD86 requires NF-κB activation and is a hallmark of B-cell stimulation enabling functional interaction with other cells or cytokines. We, therefore, measured CD25 and CD86 expression on B cells from WT and Vav1/2-/- mice after stimulation with anti-CD180. Unlike WT B cells, Vav1/2-/- B cells failed to significantly increase expression of CD25 or CD86 (Figure 5A), indicating a role for Vav proteins in this process.
Vav-deficient B cells show defects in expression of activation markers following anti-CD180 and LPS stimulation. Purified splenic B cells from WT or Vav1/2-/- mice were cultured for 26 hours and analyzed for the expression of (A) activation markers CD86 and CD25 or (B) intracellular levels of Bcl-xL. □ indicates media-only-cultured cells; ▪, CD180-stimulated cells; and ▦, LPS-stimulated cells. Error bars represent standard deviation (n = 3). Difference in increase: *P < .05; **P < .01; ***P < .001 by Student t test. MFI indicates mean fluorescence intensity.
Vav-deficient B cells show defects in expression of activation markers following anti-CD180 and LPS stimulation. Purified splenic B cells from WT or Vav1/2-/- mice were cultured for 26 hours and analyzed for the expression of (A) activation markers CD86 and CD25 or (B) intracellular levels of Bcl-xL. □ indicates media-only-cultured cells; ▪, CD180-stimulated cells; and ▦, LPS-stimulated cells. Error bars represent standard deviation (n = 3). Difference in increase: *P < .05; **P < .01; ***P < .001 by Student t test. MFI indicates mean fluorescence intensity.
We also analyzed Bcl-xL expression following anti-CD180 or LPS stimulation of B cells, as Bcl-xL is an NFκB-responsive antiapoptotic protein.25 WT B cells showed increased Bcl-xL expression in response to anti-CD180 or LPS; however, there was no increase of Bcl-xL expression in Vav1/2-/- B cells in response to anti-CD180 (Figure 5B). Furthermore the induction of Bcl-xL was significantly decreased in response to LPS. The failure of Vav1/2-/- B cells to express normal levels of Bcl-xL following LPS stimulation could be one of the factors contributing to the reduced survival and proliferation.
Discussion
The results presented here demonstrate that the Vav proteins are required for B cells to respond efficiently to LPS. The data support the hypothesis that Vav proteins are involved in signaling pathways downstream of CD180 and TLR4 in B cells. CD180 signaling, which is B-cell specific, was very sensitive to loss of Vav1. As the CD180 pathway additionally involves CD19 and BTK, it might provide B cells with a mechanism for integrating LPS responses with signals from the antigen receptor and CD19 complex.
Prepublished online as Blood First Edition Paper, April 5, 2005; DOI 10.1182/blood-2004-10-3919.
Supported by the Biotechnology and Biological Sciences Research Council (BBSRC) and Medical Research Council (MRC). M.T. holds a Medical Research Council Senior Non-Clinical Fellowship.
B.H. and E.V. contributed equally to this manuscript.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Helen Reynolds and barrier facility staff for technical assistance and members of the Turner group and Klaus Okkenhaug for helpful comments.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal