Abstract
Cluster of differentiation (CD) antigens are expressed on cells of myeloid and lymphoid lineages. As most disease processes involve immune system activation or suppression, these antigens offer unique opportunities for monitoring host responses. Immunophenotyping using limited numbers of CD antigens enables differentiation states of immune system cells to be determined. Extended phenotyping involving parallel measurement of multiple CD antigens may help identify expression pattern signatures associated with specific disease states. To explore this possibility we have made a CD monoclonal antibody array and scanner, enabling the parallel immunophenotyping of leukocyte cell suspensions in a single and rapid analysis. To demonstrate this approach, we used the specific example of patients infected with human immunodeficiency virus type-1 (HIV-1). An invariant HIV-induced CD antigen signature has been defined that is both robust and independent of clinical outcome, composed of a unique profile of CD antigen expression levels that are both increased and decreased relative to internal controls. The results indicate that HIV-induced changes in CD antigen expression are disease specific and independent of outcome. Their invariant nature indicates an irreversible component to retroviral infection and suggests the utility of CD antigen expression patterns in other disease settings.
Introduction
Protein expression at the cell surface is influenced by multiple factors including cell type, differentiation state, and pathological intracellular and extracellular processes. Whereas the expression of individual proteins changes in different situations, it is likely that alterations in the expression of whole collections of both related and unrelated proteins at cell surfaces may change in a correlated manner. These linked changes in protein expression may provide greater diagnostic power than any individual measurement, obtained for example by flow cytometry. Whereas the first monoclonal antibodies were directed against sheep red-cell antigens,1,2 it became clear that the hybrid method could be extended to the phenotyping of leukocyte cell surfaces,3 and that such phenotyping had clinical as well as biological utility.4-6 The leukocyte cell-surface antigens, which are expressed on virtually every cell type, came to be known as CD antigens on the basis of the cluster analysis statistical method used to define them.7-10
In the introduction to the first Human Leukocyte Differentiation Antigen (HLDA) workshop on CD antigens, the innovator of monoclonal antibody technology César Milstein speculated that the “complete definition of such mosaics” on cell surfaces would be “invaluable in defining cells of varying differentiation and pathological states.”11 Although the definition of the expression levels of limited numbers of CD antigens has been of great importance, the intrinsic limitations of conventional cytofluorometric methods have prevented the realization of Milstein's extended cell-surface phenotyping proposition. We have used multiplexing technology to construct a CD monoclonal antibody array (the LD array; see “Acknowledgment”) that makes the parallel expression analysis of multiple cell surface CD antigens feasible, and offers the potential of enabling the complete cell surface mosaic to be defined.
The direct profiling of protein expression levels has significant advantages over gene expression analysis, as the abundance of mRNA does not necessarily reflect the levels of mature protein product.12 Parallel expression profiling consequently provides a more accurate picture of CD antigen cell-surface abundance and could be extended to include the immunoprofiling of intracellular and soluble CD antigens. The determination of CD antigen expression profiles represents a qualitatively different approach to cell-surface immunophenotyping, in that information is extracted not just from the expression of individual molecules but from the entire surface CD antigen expression pattern. CD antigen fingerprinting in a higher dimensional space made possible with our approach provides significant advantages over the unitary measurements obtained using conventional methods. We expect the approach to be further extended by the accumulation of large databases of CD antigen patterns and the application of pattern-recognition software. Our study provides the first example of the application of such monoclonal antibody array methodologies to cells derived ex vivo from human immunodeficiency virus type-1 (HIV-1)–infected individuals. An improved understanding of this retrovirus, which infects 60 million people worldwide, with the accompanying promise of new therapeutic approaches constitutes one of the greatest medical challenges of the 21st century.13,14
In order to obtain extended CD antigen cell-surface immunophenotypes, mononuclear leukocytes are captured onto robotically dispensed monoclonal antibody dots. The number of cells captured at each site is proportional to the frequency of the cells in the test population expressing the CD antigen against which the immobilized monoclonal antibody has been raised. We have used this CD monoclonal antibody array to demonstrate the feasibility of the extended CD antigen immunophenotyping strategy by generating CD antigen expression “fingerprints.” The general utility of this approach is illustrated by the identification of a remarkably conserved HIV-induced cell-surface CD antigen expression pattern, which is found to persist in an invariable manner in a clinically heterogeneous group of HIV-infected individuals.
Study design
Patients
All the patients in the study were recruited from the Chelsea and Westminster Hospital, London, and provided written informed consent. LTNPs (long term nonprogressors) had been diagnosed as HIV-1+ for a duration of more than 10 years, had never received antiretroviral treatment, and consistently maintained a CD4 count of greater than 400 cells/mm3. Individuals undergoing first-line therapy were previously treatment naive and had elevated HIV-1 viral loads. They were recruited into the Trizivir induction maintenance study (TIMS) (manuscript submitted) and had subsequently received combination (efavirenz and Combivir) therapy. Those patients receiving salvage therapy have been previously described in the RESTART (randomized trial to investigate the recycling of stavudine and didanosine) study.15 They had received a median of 4 previous lines of antiretroviral therapy with nonnucleoside reverse transcriptase or protease inhibitor–based regimens and at the time of blood draw and freezing, were receiving the nucleoside analogs stavudine and didanosine. Normal control samples were obtained from healthy volunteers and the study received appropriate ethical approval from the Chelsea and Westminster Hospital Institutional Review Board.
Microarray construction
A PixSys 3200 Aspirate and Dispense System (Cartesian Technologies, Irvine, CA) was used to construct duplicate microarrays consisting of 87 different 10-nL antibody dots immobilized on a film of nitrocellulose (18 × 27 mm) deposited onto the surface of a glass microscope slide (FAST Slides; Grace Biolabs, Bend, OR, supplied by Schleicher and Schuell, Keene, NH). Monoclonal antibodies were purchased from the following companies: Coulter and Immunotech from Beckman Coulter (Gladesville, New South Wales, Australia), Pharmingen from BD Biosciences (North Ryde, New South Wales, Australia), Biosource International from Monarch Medical (Stafford City, Queensland, Australia), Serotec from Australian Laboratory Services (Sydney Markets, New South Wales, Australia), Sigma-Aldrich (Castle Hill, New South Wales, Australia). Antibody solutions were reconstituted as recommended by the suppliers, frozen in aliquots at -20°C with 0.1% (wt/vol) bovine serum albumin (BSA; Sigma-Aldrich), and used at concentrations that ranged from 50 to 1000 μg/mL.
Following robotic deposition of the antibody dots (10 nL) in a rectangular array, the nitrocellulose was blocked with 5% (wt/vol) skim milk (Diploma; Bonlac Foods, Melbourne, Victoria, Australia) in phosphate-buffered saline (PBS; 90 minutes at room temperature, or overnight at 4°C), washed twice with water, dried, and stored at 4°C with desiccant. Each batch of slides was tested with cell lines, frozen peripheral blood mononuclear cells, or leukemia cells of known immunophenotype to check their profile of antibody-binding activities.
Leukocyte binding
Mononuclear cells were obtained using the well-described Ficoll-Histopaque density gradient separation methods and cryopreserved in fetal calf serum (FCS)/10% dimethylsulfoxide (Sigma, Poole, United Kingdom). These were rapidly thawed and washed in PBS. Mononuclear cells (4-6 × 106 cells) were suspended in 300 μL PBS with added heat-inactivated human AB antigen-positive serum and incubated at room temperature (20°C) for 45 minutes. Unbound cells were gently removed by washing with PBS and the arrays fixed for at least 20 minutes in PBS containing 3.7% (wt/vol) formaldehyde before washing with PBS again.
Data recording
Mononuclear cells captured by immobilized CD monoclonal antibody dots on the LD array were imaged using a Medsaic Slide Reader and DotScan software as described previously.16 This quantifies the intensity of captured cells on each antibody dot from the digital image file and compiles bar charts that show the average intensities above background on an 8-bit scale ranging from 1 to 256.
The digital images obtained from dot patterns from several experiments were analyzed both by ScanTalk and ImageQuant 5.1 (Amersham Biotech, Amersham, United Kingdom). A comparison of extensive dot-intensity data sets showed a linear relationship between the values obtained using these 2 different software packages. There was also a linear relationship between the number of mononuclear leukocytes captured on each CD monoclonal antibody dot (10 nL) and the intensity of the dot as measured using ScanTalk (with approximately 800 cells saturating the dot). Previous comparisons of data obtained with the LD Array (ScanTalk intensity), with analysis of the same cell samples using flow cytometry and measuring either mean fluorescence intensity per cell or percentage of positive cells, demonstrated a close correlation between the 2 procedures.
Statistical methods
Before analyzing the data, a number of preprocessing steps were implemented. First, the raw intensity measurements on a chip, denoted by CDij (where i = L,R denotes whether the measurement is taken from the left-hand or right-hand side of the chip and j represents the antigen), were transformed using the variance-stabilizing square-root transformation (ie,
The normalized data described above were then analyzed using the R statistical language and environment (http://www.r-project.org). The R-contributed libraries “limma” (http://bioinf.wehi.edu.au/limma/) and “mult-test” (http://www.bioconductor.org/) provided the necessary routines for fitting linear models to microarray data that incorporate appropriate empirical Bayes smoothing of variances and for making multiple testing adjustments using the false discovery rate.19-21 Modified F tests and moderated t tests were used to identify antigens that were differentially expressed overall, both among and pairwise between the 4 patient groups (ie, LTNPs, healthy, salvage therapy, and the first-line therapy). We used an adjusted (for multiple testing) P value of less than .005 to identify differential expression. The adjusted P values were calculated based on a step-up procedure that controls the false discovery rates.22,23 A P value of .005 was chosen because this study is limited by small sample sizes. The conclusions are in general unchanged if the criterion used to identify differential expression is the (log) posterior odds of being differentially expressed (ie, the B statistics produced by limma). The results described in this paper are as a consequence based on the adjusted P values.
Results and discussion
The pathogenic mechanisms underlying HIV infection are heterogeneous, with the clinical outcome depending on a complex interplay between host and pathogenic factors. In untreated individuals, the median time from infection with virus to development of AIDS is 10 years.24,25 HIV-infected patients require antiretroviral treatment to suppress viremia and to maintain CD4 counts. A minority of HIV-infected individuals, known as long-term nonprogressors (LTNPs), remain healthy for more than 10 years with no clinical evidence of disease progression. To further investigate the extended CD antigen expression patterns on the cell surfaces of HIV-infected individuals, we constructed an LD array containing 84 different CD monoclonal antibody spots (representing around a quarter of the 339 currently defined CD antigens) and antibodies against a number of control antigens, including human leukocyte antigen (HLA)–DR and T-cell receptors (TCRs). The LD array was then used to determine the extended cell-surface CD antigen immunophenotype of mononuclear cells derived from healthy subjects (n = 4), LTNPs (n = 6), HIV-infected individuals undergoing first-line antiretroviral therapy (n = 10), and extensively pretreated individuals with advanced HIV disease who were undergoing salvage therapy (n = 6). Table 1 outlines the CD4 count and viral load profiles of all of the individuals recruited into this study.
Patient characteristics
. | LTNPs, no treatment required . | First-line patients, given highly active antiretroviral therapy . | Salvage therapy patients, given HAART following at least 4 previous courses . |
|---|---|---|---|
| CD4 count, median cells/mm3 (interquartile range) | 701 (447-793) | 194 (94-261) | 78 (48-107) |
| HIV viral load, median copies/mL (interquartile range) | 640 (< 50-1826) | 1487 (84-44 910) | 103 090 (53 246-361 326) |
. | LTNPs, no treatment required . | First-line patients, given highly active antiretroviral therapy . | Salvage therapy patients, given HAART following at least 4 previous courses . |
|---|---|---|---|
| CD4 count, median cells/mm3 (interquartile range) | 701 (447-793) | 194 (94-261) | 78 (48-107) |
| HIV viral load, median copies/mL (interquartile range) | 640 (< 50-1826) | 1487 (84-44 910) | 103 090 (53 246-361 326) |
CD4 counts were measured by staining whole blood with murine anti-human monoclonal antibodies to CD4 (TetraOne; Beckman Coulter, High Wycombe, United Kingdom) with analysis on an Epics XL-MCL (Beckman Coulter) flow cytometer. Viral loads in patient plasma was measured using the Quantiplex HIV RNA 3.0 (Chiron bDNA) assay with a lower limit of detection of 50 HIV-1 copies/mL (Chiron Diagnostics, Halstead, United Kingdom).
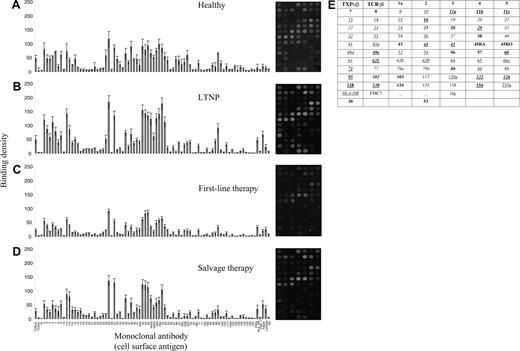
Representative antibody response charts and binding patterns of mononuclear cells on the LD array. (A) Healthy individuals, (B) long-term nonprogressors, (C) first-line therapy, and (D) salvage therapy. The median and standard error of the mean of the binding intensity of each CD antigen is shown. Labeling on the x-axis refers to monoclonal antibodies with specificities against the corresponding CD antigens; TCRαβ, TCRγδ, HLA-DR, FMC-7, κ, λ, and sIg are monoclonal antibodies against T-cell receptors αβ and γδ, HLA-DR, FMC-7, kappa and lambda immunoglobulin light chains, and surface immunoglobulin, respectively. On the right of each histogram, corresponding dot plots demonstrate the binding patterns of mononuclear cells on the LD array. (E) The key for these CD antigens in the dot plot is as follows: boldface indicates T-cell markers; italics, B-cell markers; and underline, myeloid markers. Combinations of these indicate lineage origin. Others are left in normal font; stem cell markers were CD34 and CD117.
Representative antibody response charts and binding patterns of mononuclear cells on the LD array. (A) Healthy individuals, (B) long-term nonprogressors, (C) first-line therapy, and (D) salvage therapy. The median and standard error of the mean of the binding intensity of each CD antigen is shown. Labeling on the x-axis refers to monoclonal antibodies with specificities against the corresponding CD antigens; TCRαβ, TCRγδ, HLA-DR, FMC-7, κ, λ, and sIg are monoclonal antibodies against T-cell receptors αβ and γδ, HLA-DR, FMC-7, kappa and lambda immunoglobulin light chains, and surface immunoglobulin, respectively. On the right of each histogram, corresponding dot plots demonstrate the binding patterns of mononuclear cells on the LD array. (E) The key for these CD antigens in the dot plot is as follows: boldface indicates T-cell markers; italics, B-cell markers; and underline, myeloid markers. Combinations of these indicate lineage origin. Others are left in normal font; stem cell markers were CD34 and CD117.
The cell surface CD antigen mosaic for each group and accompanying dot pattern from which the raw data were derived is shown in Figure 1. There were no cell-surface molecules on LTNPs whose expressions differed significantly from those on the cell surfaces of the other 2 HIV-infected groups (first-line therapy and salvage therapy). Specifically, we did not find a single CD antigen whose expression was significantly different in pairwise comparisons across all 3 HIV-infected groups that we studied (this may be due to a lack of power because of small numbers in each infected group). In pairwise comparisons (Table 2), we consistently observed that CD60, CD102, and CD126 expression was decreased in all of the HIV-infected groups compared with healthy controls (P < .001). For all 3 HIV-infected groups versus healthy controls, we also observed that the expression of HLA-DR and CD20 was significantly increased (P < .004). The conservation of cell-surface CD antigen markers in HIV infection is further suggested by data obtained in the overall comparison among the 4 groups, which demonstrates that 38 out of the 84 measured antigens had adjusted P values of approximately .2 and over (ie, trendless nonsignificant data). This is supported by previous studies, all of which have failed to identify host factors that mediate the benign but clinically important (LTNP) outcome in humans. Such data, however, do not always compare directly with flow cytometric results in which only small numbers of cell-surface markers are compared in parallel using straightforward parametric or nonparametric tests, and we emphasize that we are not undertaking flow cytometry on a chip. Many of the markers that we study herein have not been studied with internal HIV-1+ and HIV-1- controls and with the same rigorous statistical criteria applied to multiplexing (analogous to cDNA microarray analyses). To illustrate this, while CD4 data are clearly higher in healthy and LTNP patients in our analysis (see Figure 1), as they should be, we did not find these differences to be statistically significant. In addition, the CD array and scanning system that we used may not be optimal for the quantitative detection of every CD antigen as binding conditions may be linear for some antigens and nonlinear for others under the same sample preservation and assay testing conditions. The acceptance of suboptimality of some data points is an intrinsic constraint of the multiplexing approach, although in approaches such as ours that rely on pattern recognition, the absolute values of individual data points are of less consequence than the overall nature of the expression “fingerprint.”
Statistically different levels of CD antigen expression
Antigen . | Relative expression . | Adjusted P . |
|---|---|---|
| Healthy vs LTNP | ||
| Decreased in LTNP | ||
| CD60 | 4.87 | < .001 |
| CD30 | 4.47 | .003 |
| CD102 | 3.58 | < .001 |
| CD126 | 2.47 | < .001 |
| Increased in LTNP | ||
| HLA-DR | – 3.71 | .004 |
| CD20 | – 2.32 | < .001 |
| TCRγδ | – 1.19 | .002 |
| Salvage therapy vs healthy | ||
| Decreased in salvage therapy | ||
| CD60 | – 5.29 | < .001 |
| CD28 | – 4.27 | .001 |
| CD102 | – 3.70 | < .001 |
| CD126 | – 2.81 | < .001 |
| CD30 | – 1.42 | < .001 |
| Increased in salvage therapy | ||
| HLA-DR | 4.18 | .004 |
| CD11b | 3.83 | < .001 |
| CD20 | 3.09 | < .001 |
| TCRγδ | 1.06 | .002 |
| First-line therapy vs healthy | ||
| Decreased in first-line therapy | ||
| CD60 | – 4.75 | < .001 |
| CD102 | – 2.98 | < .001 |
| CD126 | – 2.52 | < .001 |
| CD130 | – 2.08 | .001 |
| CD43 | – 1.92 | .003 |
| Increased in first-line therapy | ||
| HLA-DR | 4.84 | < .001 |
| CD20 | 3.09 | < .001 |
Antigen . | Relative expression . | Adjusted P . |
|---|---|---|
| Healthy vs LTNP | ||
| Decreased in LTNP | ||
| CD60 | 4.87 | < .001 |
| CD30 | 4.47 | .003 |
| CD102 | 3.58 | < .001 |
| CD126 | 2.47 | < .001 |
| Increased in LTNP | ||
| HLA-DR | – 3.71 | .004 |
| CD20 | – 2.32 | < .001 |
| TCRγδ | – 1.19 | .002 |
| Salvage therapy vs healthy | ||
| Decreased in salvage therapy | ||
| CD60 | – 5.29 | < .001 |
| CD28 | – 4.27 | .001 |
| CD102 | – 3.70 | < .001 |
| CD126 | – 2.81 | < .001 |
| CD30 | – 1.42 | < .001 |
| Increased in salvage therapy | ||
| HLA-DR | 4.18 | .004 |
| CD11b | 3.83 | < .001 |
| CD20 | 3.09 | < .001 |
| TCRγδ | 1.06 | .002 |
| First-line therapy vs healthy | ||
| Decreased in first-line therapy | ||
| CD60 | – 4.75 | < .001 |
| CD102 | – 2.98 | < .001 |
| CD126 | – 2.52 | < .001 |
| CD130 | – 2.08 | .001 |
| CD43 | – 1.92 | .003 |
| Increased in first-line therapy | ||
| HLA-DR | 4.84 | < .001 |
| CD20 | 3.09 | < .001 |
The expression of a number of antigens was found to be significantly different between the groups. Relative expression refers to the difference in the average normalized intensities between the 2 groups. We observed no statistically significant differences between LTNP and first-line therapy and between LTNP and salvage therapy. For salvage therapy versus first-line treatment, CD135 was decreased in salvage therapy (relative expression - 1.3; P < .001). Other antigens, including some contained on this array, have been previously demonstrated (by flow cytometry) to be up- or down-regulated at different stages of infection, and we do not find statistical significance with many of these. While these data demonstrate discrepancies with such findings, we are performing statistical comparisons across 4 distinct infected groups. Further assays will involve a greater number of CD antigens and it is anticipated that the array itself could be subdivided into smaller subarrays such that linear antigen binding conditions and dynamics could be optimized for a greater number of CD antigens.
Many changes in cell-surface CD antigen expression demonstrated herein have not previously been documented, and demonstrate the intrinsic multiplexing power of the LD array and its ability to define cell-surface CD antigen fingerprints in a single test. They also suggest the importance and clinical utility of the parallel-profiling of multiple cell-surface CD antigens and of comparing these CD antigen mosaics in different clinical contexts, though confirmation is required by other assays. Most strikingly though, the results show that all HIV-infected mononuclear cells share a cell-surface mosaic that clearly distinguishes them from normal cells, and which arises and persists in a manner that is independent of the overall clinical outcome.
It appears that the HIV infection event induces a conserved and apparently irreversible programmed change in the expression of a discreet set of cell-surface CD antigens. Considering the large number of CD antigens measured, we were surprised to observe that the cell surface phenotypic response of mononuclear cells to infection with HIV was conserved in individuals with diverse clinical outcomes. Changes in the cell-surface expression of CD60, CD102, CD126 (down-regulated in HIV infection), HLA-DR, and CD20 (up-regulated in HIV infection) appear to be robust, invariant, homogenous, and fully independent of host factors. The functional significance of these findings requires further investigation, but the overexpression of the CD20 antigen on the surface of the mononuclear cells of HIV-infected individuals, for example, suggests that increased levels of surface CD20 may contribute to the increased incidence of CD20+ lymphomas in HIV-positive populations.26 The finding of increased levels of HLA-DR in cells derived from HIV-infected patients has been well documented.27,28
It appears then that, for the greater part, viral and not host factors determine clinical outcome following HIV infection. This finding is consistent with the numerous reported viral factors (eg, gene deletions or defects that have been reported in HIV genes and the long terminal repeat) that have been associated with nonprogression.29,30 In contrast, there is a distinct lack of consistently reported and reproducible host factors (other than the rare occurrence of a single copy of the chemokine receptor-5 [CCR-5] Δ32 receptor mutation31 ) that correlate with retroviral disease outcome.
The method we describe has the following benefits: (1) it has the potential to produce a comprehensive description of the entire cell surface in a single test, compared with flow-cytometric analyses which enable a maximum of several antigens to be simultaneously assessed; (2) the approach holds the promise of a new paradigm for clinical medicine based upon the use of patterns of cell-surface antigens to identify disease states; (3) the method allows for the subcategorization of cellular differentiation states by allowing the simultaneous and parallel determination of the expression values for the entire set of cell-surface CD antigens; (4) it provides new clues for studying perturbation of host immunity, pathogenesis, and disease progression; (5) it should be generalizable to intracellular CD antigens and indeed even to soluble CD antigens (although the assay would need to be configured slightly differently to detect these); (6) most important, it represents the apotheosis of the entire CD antigen concept/program as originally defined by César Milstein at the outset32 ; and (7) it should help identify new CD antigen therapeutic targets. This has never been done before and is a major conceptual achievement that has great promise for clinical medicine as it appears likely that in some if not many disease states, it will be possible to define expression signatures that do vary with clinical outcome, indicating that this approach could be of both diagnostic and prognostic significance.
In conclusion and according to this specific methodology, our results suggest the general utility of extended parallel cell-surface CD antigen immunoprofiling using the LD monoclonal antibody array in helping to define cell-surface mosaics that may be of diagnostic, prognostic, and therapeutic significance. The accumulation of large databases of such CD antigen patterns and the identification through appropriate statistical methods of disease specific patterns may provide the basis of a new paradigm based on the determination of complete profiles of antigen expression, provided that independent confirmation in other assay arenas validates these data.
Prepublished online as Blood First Edition Paper, April 12, 2005; DOI 10.1182/blood-2004-12-4642.
J.C. is the CEO of Medsaic Pty Ltd, which provided all of the CD antibody microarrays used in this study. R.C. is a Director of Medsaic and holds shares in the company.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The LD array is a microarray of monoclonal CD antibodies named in memory of Mrs Lee Dixon.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal