Abstract
A proportion of cancer patients naturally develop CD4+ T-helper type 1 (Th1) cell responses to NY-ESO-1 that correlate with anti–NY-ESO-1 serum antibodies. To address the role of T-cell regulation in the control of spontaneous tumor immunity, we analyzed NY-ESO-1–specific Th1 cell induction before or after depletion of CD4+CD25+ T cells in vitro. While Th1 cells were generated in the presence of CD25+ T cells in cancer patients seropositive for NY-ESO-1, seronegative cancer patients and healthy donors required CD25+ T-cell depletion for in vitro induction of NY-ESO-1–specific Th1 cells. In vitro, newly generated NY-ESO-1–specific Th1 cells were derived from naive precursors, whereas preexisting memory populations were detectable exclusively in patients with NY-ESO-1 antibody. Memory populations were less sensitive than naive populations to CD4+CD25+ regulatory T cells. We propose that CD4+CD25+ regulatory T cells are involved in the generation and regulation of NY-ESO-1–specific antitumor immunity.
Introduction
NY-ESO-1 is a germ-cell protein that is often expressed by cancer cells, but not by normal somatic cells.1 In monitoring a large series of cancer patients, frequent humoral responses to NY-ESO-1 were found and they correlated with NY-ESO-1 expression in tumor cells and with the presence of peripheral CD8+ T cells against NY-ESO-1,2,3 suggesting the involvement of CD4+ helper T cells in coordinating this response. In fact, we have confirmed that NY-ESO-1–specific CD4+ T helper cells are only found in NY-ESO-1 seropositive patients, but not in seronegative patients or healthy individuals.4
Naturally occurring CD4+CD25+ regulatory T cells play an important role in maintaining immunologic balance in hosts by suppressing a wide variety of immune responses.5-8 Although this T-cell population was originally found to suppress the development of autoimmunity,5 it has been shown that depletion of this regulatory T-cell population by antibody enhances the antitumor immune responses9,10 and that stimulation of CD4+CD25+ regulatory T cells by immunization with self-antigens exacerbates tumor growth in mouse studies.11 In human cancer settings, CD4+CD25+ regulatory T cells exist at the local tumor site and contribute to growth of tumors in vivo and are associated with an unfavorable prognosis.12-15
In contrast to our previous data, it has recently been reported that autoreactive T-cell precursors to NY-ESO-1 are present in most healthy individuals, and that CD4+CD25+ regulatory T cells play an important role for controlling their activation.16 These observations prompted us to extend this analysis to cancer patients and assess the impact of CD4+CD25+ regulatory T cells on the generation of NY-ESO-1–specific CD4+ T-cell responses in relation to NY-ESO-1 expression in the tumor and presence of specific serum antibody.
Study design
Donor samples
All healthy donors were subjects with no history of autoimmune disease. All cancer patients had NY-ESO-1 expressing melanoma except patients NW2493 with small cell lung cancer and NW1060 with sarcoma. All samples were collected after informed consent as part of a study approved by the Ethics Committee of Landesärztekammer Hessen, Frankfurt.
Antibodies and reagents
Tri-Color–conjugated anti-CD4 and anti-CD45RA and fluorescein isothiocyanate–conjugated anti-CD45RO antibodies were purchased from CALTAG (Burlingame, CA). R-phycoerythrin–conjugated anti-CD25 antibody was purchased from Miltenyi Biotec (Auburn, CA). Synthetic peptides of NY-ESO-187-98 (LLEFYLAMPFAT), NY-ESO-1121-132 (VLLKEFTVSGNI), NY-ESO-1143-154 (RQLQLSISSCLQ), NY-ESO-1157-170 (SLLMWITQCFLPVF), and HIV P1737-51 (ASRELERFAVNPGLL)4 were obtained from Bio Synthesis (Lewisville, TX). All peptides were shown to be restricted by HLA-DRB1*04 or HLA-DRB1*07, except NY-ESO-1157-170 restricted by HLA-DPB1*04.4,17
Generation of NY-ESO-1–specific CD4+ T cells
NY-ESO-1–specific CD4+ T cells were elicited as described previously with some modifications.4,16 Briefly, CD4+ T cells and CD4+CD25- T cells were isolated from peripheral blood mononuclear cells (PBMCs) using CD4+CD25+ Regulatory T-Cell Isolation Kit (Miltenyi Biotec). In some experiments, CD4+CD25- T cells (> 96% purity) were further separated into CD45RO-depleted T cells (CD4+CD25-CD45RA+ T cells) (> 96% purity) or CD45RA-depleted T cells (CD4+CD25-CD45RO+ T cells) (> 93% purity) using CD45RO or CD45RA Microbeads (Miltenyi Biotec), respectively. Antigen-presenting cells (APCs) were prepared from non-CD4+ cells by allowing them to adhere to tissue culture plates (Corning Inc, Corning, NY) for 2 hours, removing nonadherent cells, and pulsing them with 10 μM of 1 or 2 peptides overnight. After irradiation, 1 × 105 APCs were added to round-bottom 96-well plates (Corning) containing 5 × 104 unfractionated CD4+, CD4+CD25-, CD4+CD25-CD45RO+, or CD4+CD25-CD45RA+ T cells and were fed with 10 U/mL interleukin (IL)–2 (Roche Molecular Biochemicals, Indianapolis, IN) twice per week.
Enzyme-linked immunospot assay
The number of interferon (IFN)–γ–secreting peptide-specific CD4+ T cells was assessed by enzyme-linked immunospot (ELISPOT) assays against phytohaemagglutinin (PHA HA15; Murex Diagnostics, Dartford, United Kingdom)–activated CD4+ T cells or Epstein Barr virus–transformed B cells pulsed with 10 μM of peptides overnight, as described previously.4,18
Proliferation assay
Presensitized T cells (5 × 104) were cultured with 5 × 104 irradiated CD3-depleted PBMCs pulsed with 10 μM of peptides overnight in wells of round-bottom 96-well plates. Proliferation was evaluated by pulsing with 0.037 MBq/well (1 μCi/well) [3H]-thymidine for the last 18 hours of a 72-hour culture. [3H]-thymidine incorporation was measured by a scintillation counter.
Results and discussion
NY-ESO-1–specific CD4+ T-cell precursors are present in healthy donors
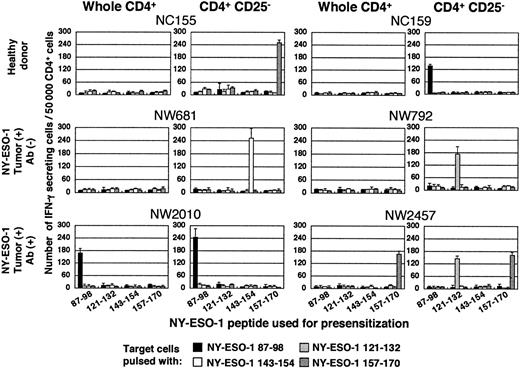
CD4+ T cells and CD4+CD25- T cells were isolated from PBMCs and were cultured with APCs pulsed with a series of HLA class II–restricted NY-ESO-1 peptides reported previously.4 Fifteen to 20 days later, NY-ESO-1–specific CD4+ helper T-cell induction was analyzed by ELISPOT (Figure 1) and proliferation (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article) assays. IFN-γ–secreting NY-ESO-1–specific CD4+ T cells with proliferative capacity, CD4+ T helper type 1 (Th1) cells, were elicited in 5 of 8 healthy donors, but only in cultures with CD4+CD25+ T-cell depletion, confirming a previous report.16
NY-ESO-1–specific CD4+ T cell precursors are present not only in patients with NY-ESO-1 antibody but also in patients without NY-ESO-1 antibody. CD4+ T cells and CD4+CD25- T cells were isolated from PBMCs as described in “Study design” and were cultured with APCs pulsed with indicated NY-ESO-1 peptides. Fifteen to 20 days later, NY-ESO-1–specific Th1 cell induction was analyzed by ELISPOT assay. These experiments were performed independently at least twice with similar results. Data are expressed as means +SD.
NY-ESO-1–specific CD4+ T cell precursors are present not only in patients with NY-ESO-1 antibody but also in patients without NY-ESO-1 antibody. CD4+ T cells and CD4+CD25- T cells were isolated from PBMCs as described in “Study design” and were cultured with APCs pulsed with indicated NY-ESO-1 peptides. Fifteen to 20 days later, NY-ESO-1–specific Th1 cell induction was analyzed by ELISPOT assay. These experiments were performed independently at least twice with similar results. Data are expressed as means +SD.
NY-ESO-1–specific CD4+ T-cell precursors are present in cancer patients with and without NY-ESO-1 antibody
Next, we extended this examination to patients with NY-ESO-1–expressing tumors. Th1 cells were induced in 3 of 3 patients with NY-ESO-1 antibody, without need for CD4+CD25+ T-cell depletion in accordance with our previous results.4 In patients without NY-ESO-1 antibody, although NY-ESO-1–specific Th1 cells were not induced from total CD4+ T-cell populations, they could be induced in 3 out of 4 NY-ESO-1 seronegative patients after depletion of CD4+CD25+ T cells (Figures 1 and S1, summarized in Table 1). Taken together, NY-ESO-1–specific CD4+ T-cell precursors are present in a wider range of patients than formerly thought.4
Summary of generation of NY-ESO-1–specific CD4+ T helper cells
. | . | NY-ESO-1 (peptide-specific IFN-γ-secreting cells/ratio of proliferation) . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | HLA class II DRB1 alleles . | 87–98 . | . | 121–132 . | . | 143–154 . | . | 157–170 . | . | |||||||
. | . | Whole CD4 . | CD4+ CD25- . | Whole CD4 . | CD4+ CD25- . | Whole CD4 . | CD4+ CD25- . | Whole CD4 . | CD4+ CD25- . | |||||||
| Healthy donors | ||||||||||||||||
| NC78 | 04,11 | ND | ND | – (18/1.3) | + (212/3.3) | ND | ND | ND | ND | |||||||
| NC155 | 04,11 | – (7/1.2) | – (12/1.1) | – (14/1.1) | – (16/0.9) | – (7/0.9) | – (16/0.8) | – (18/1.0) | + (251/3.1) | |||||||
| NC158 | 04,04 | – (5/1.1) | – (14/1.0) | – (12/1.1) | – (8/0.9) | – (11/1.0) | – (6/1.2) | ND | ND | |||||||
| NC159 | 03,04 | – (10/0.8) | + (137/4.1) | – (10/1.0) | – (5/1.3) | – (13/0.9) | – (9.5/0.8) | – (9/0.9) | – (8/1.0) | |||||||
| NC174 | 04,07 | ND | ND | – (11/1.1) | – (5/0.9) | ND | ND | ND | ND | |||||||
| NC185 | 04,07 | – (12/1.0) | + (152/3.8) | – (7/1.0) | – (9/1.2) | – (11/1.0) | – (6/1.1) | – (7/0.9) | – (12/1.0) | |||||||
| NC198 | 07,09 | – (14/0.8) | + (243/3.5) | – (9/1.1) | – (13/1.2) | – (11/1.0) | – (10/1.1) | ND | ND | |||||||
| NC233 | 04,04 | – (7/1.0) | – (14/1.2) | – (10/0.8) | – (10/0.9) | – (9/1.0) | – (15/1.2) | – (13/1.1) | – (9.5/1.1) | |||||||
| NY-ESO-1 tumor+ Ab- patients | ||||||||||||||||
| NW681 | 13,13 | – (10/0.8) | – (16/0.9) | – (11/1.3) | – (8/1.3) | – (17/1.1) | + (252/3.8) | – (18/1.2) | – (12/0.9) | |||||||
| NW792 | 04,15 | – (17/1.2) | – (20/1.3) | – (12/0.7) | + (173/5.0) | – (19/1.2) | – (14/1.9) | – (11/0.9) | – (13/0.8) | |||||||
| NW1028 | 04,15 | – (6/1.3) | – (9/1.2) | – (11/0.9) | – (10/1.0) | – (11/0.8) | – (8/0.9) | – (9/1.6) | + (201/4.1) | |||||||
| NW1060 | ND | – (7/1.2) | – (11/0.9) | – (10/1.4) | – (7/0.8) | – (9/1.1) | – (10/1.3) | – (5/1.0) | – (13/1.1) | |||||||
| NY-ESO-1 tumor+ Ab+ patients | ||||||||||||||||
| NW2010 | 07,11 | + (168/13) | + (244/4.5) | – (9/1.4) | – (13/0.7) | – (6/1.8) | – (8/0.8) | – (8/1.0) | – (4/1.6) | |||||||
| NW2457 | 04,15 | – (8/1.1) | – (7/1.5) | – (9/0.7) | + (145/3.9) | – (11/1.3) | – (9/1.0) | + (165/4.8) | + (162/7.0) | |||||||
| NW2493 | 07,13 | – (8/1.1) | – (9/1.2) | + (187/3.8) | + (256/5.2) | – (8/1.3) | – (10/1.2) | – (10/1.1) | – (9/1.4) | |||||||
. | . | NY-ESO-1 (peptide-specific IFN-γ-secreting cells/ratio of proliferation) . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | HLA class II DRB1 alleles . | 87–98 . | . | 121–132 . | . | 143–154 . | . | 157–170 . | . | |||||||
. | . | Whole CD4 . | CD4+ CD25- . | Whole CD4 . | CD4+ CD25- . | Whole CD4 . | CD4+ CD25- . | Whole CD4 . | CD4+ CD25- . | |||||||
| Healthy donors | ||||||||||||||||
| NC78 | 04,11 | ND | ND | – (18/1.3) | + (212/3.3) | ND | ND | ND | ND | |||||||
| NC155 | 04,11 | – (7/1.2) | – (12/1.1) | – (14/1.1) | – (16/0.9) | – (7/0.9) | – (16/0.8) | – (18/1.0) | + (251/3.1) | |||||||
| NC158 | 04,04 | – (5/1.1) | – (14/1.0) | – (12/1.1) | – (8/0.9) | – (11/1.0) | – (6/1.2) | ND | ND | |||||||
| NC159 | 03,04 | – (10/0.8) | + (137/4.1) | – (10/1.0) | – (5/1.3) | – (13/0.9) | – (9.5/0.8) | – (9/0.9) | – (8/1.0) | |||||||
| NC174 | 04,07 | ND | ND | – (11/1.1) | – (5/0.9) | ND | ND | ND | ND | |||||||
| NC185 | 04,07 | – (12/1.0) | + (152/3.8) | – (7/1.0) | – (9/1.2) | – (11/1.0) | – (6/1.1) | – (7/0.9) | – (12/1.0) | |||||||
| NC198 | 07,09 | – (14/0.8) | + (243/3.5) | – (9/1.1) | – (13/1.2) | – (11/1.0) | – (10/1.1) | ND | ND | |||||||
| NC233 | 04,04 | – (7/1.0) | – (14/1.2) | – (10/0.8) | – (10/0.9) | – (9/1.0) | – (15/1.2) | – (13/1.1) | – (9.5/1.1) | |||||||
| NY-ESO-1 tumor+ Ab- patients | ||||||||||||||||
| NW681 | 13,13 | – (10/0.8) | – (16/0.9) | – (11/1.3) | – (8/1.3) | – (17/1.1) | + (252/3.8) | – (18/1.2) | – (12/0.9) | |||||||
| NW792 | 04,15 | – (17/1.2) | – (20/1.3) | – (12/0.7) | + (173/5.0) | – (19/1.2) | – (14/1.9) | – (11/0.9) | – (13/0.8) | |||||||
| NW1028 | 04,15 | – (6/1.3) | – (9/1.2) | – (11/0.9) | – (10/1.0) | – (11/0.8) | – (8/0.9) | – (9/1.6) | + (201/4.1) | |||||||
| NW1060 | ND | – (7/1.2) | – (11/0.9) | – (10/1.4) | – (7/0.8) | – (9/1.1) | – (10/1.3) | – (5/1.0) | – (13/1.1) | |||||||
| NY-ESO-1 tumor+ Ab+ patients | ||||||||||||||||
| NW2010 | 07,11 | + (168/13) | + (244/4.5) | – (9/1.4) | – (13/0.7) | – (6/1.8) | – (8/0.8) | – (8/1.0) | – (4/1.6) | |||||||
| NW2457 | 04,15 | – (8/1.1) | – (7/1.5) | – (9/0.7) | + (145/3.9) | – (11/1.3) | – (9/1.0) | + (165/4.8) | + (162/7.0) | |||||||
| NW2493 | 07,13 | – (8/1.1) | – (9/1.2) | + (187/3.8) | + (256/5.2) | – (8/1.3) | – (10/1.2) | – (10/1.1) | – (9/1.4) | |||||||
Italics and plus symbols indicate a significant response.
For data in parentheses, the data to the left of the slash represent spots per 50000 presensitized cells in ELISPOT assay. Background to irrelevant peptides was less than 20 spots. Data to the right of the slash repesent the ratio of proliferation to specific peptides was calculated as counts per minute (cpm) of the sample/cpm of background.
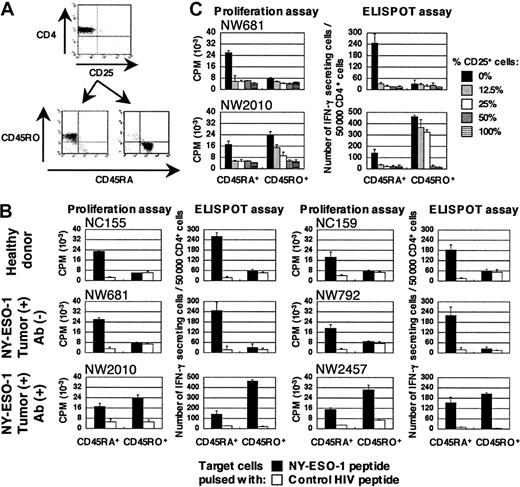
NY-ESO-1–specific CD4+ T cells are derived from distinct CD4+ T-cell populations between NY-ESO-1 seropositive and seronegative patients
We next asked whether NY-ESO-1–specific CD4+ T-cell precursors were derived from similar or from different populations in patients with or without NY-ESO-1 antibody. To address this question, CD4+CD25- T cells were further separated into naive or effector/memory populations according to typical surface-marker molecules CD45RA and CD45RO, respectively (Figure 2A). In patients with NY-ESO-1 antibody, NY-ESO-1–specific Th1 cells were induced from both CD45RA+ and CD45RO+ populations. In contrast, NY-ESO-1–specific Th1 cells were apparently derived only from CD45RA+ population in patients without NY-ESO-1 antibody and healthy donors (Figure 2B). Thus, responses elicited from depletion of CD4+CD25+ T cells are derived from a naive repertoire. Patients with preexisting immunity to NY-ESO-1 still retain the capacity to elicit additional responses from naive precursors upon CD4+CD25+ depletion (as in patient NW2457). Expression of NY-ESO-1 in the tumor is not sufficient to naturally prime NY-ESO-1–specific T-cell responses in patients without NY-ESO-1 antibody, since they have no specific precursors with memory phenotype.
CD4+CD25-CD45RO+ T-cell populations are more resistant to CD4+CD25+ regulatory T cells for NY-ESO-1–specific Th1 cell induction than CD4+CD25-CD45RA+ T-cell population
To examine the differences between patients with and without NY-ESO-1 antibody, graded amounts of CD4+CD25+ T cells were added to cultures for in vitro stimulation. NY-ESO-1–specific Th1 cells were induced from CD4+CD25-CD45RO+ T-cell population even in the presence of a high percentage of CD4+CD25+ regulatory T cells. However, induction of NY-ESO-1–specific Th1 cells from CD4+CD25-CD45RA+ T-cell population was essentially abrogated in the presence of CD4+CD25+ regulatory T cells (Figure 2C). It appears that NY-ESO-1–specific CD4+ T-cell precursors present in CD4+CD25-CD45RO+ T-cell population of patients with NY-ESO-1 antibody are more resistant to CD4+CD25+ regulatory T cells.
NY-ESO-1–specific CD4+ T cells are derived from distinct CD4+ T-cell populations showing different sensitivity to CD4+ CD25+ regulatory T cells between patients with and without NY-ESO-1 antibody. (A) CD4+CD25- T cells isolated from PBMCs were further separated into CD45RA+ or CD45RO+ using magnetic beads as described in “Study design.” (B) These T cells were cultured with APCs pulsed with appropriate NY-ESO-1 peptides according to respective HLA haplotype, namely NY-ESO-1157-170 for NC155, NY-ESO-187-98 for NC159, NY-ESO-1143-154 for NW681, NY-ESO-1121-132 for NW792, NY-ESO-187-98 for NW2010, and NY-ESO-1157-170 for NW2457, and tested for specific T-cell induction using cognate peptide or control HIV peptide by ELISPOT and proliferation assays. (C) Graded amounts of CD4+CD25+ T cells were added to cultures during in vitro peptide stimulation of NW681 and NW2010 and specific T-cell induction was examined using cognate peptides by ELISPOT and proliferation assays. These experiments were performed independently at least twice with similar results. Data are expressed as means +SD. CPM indicates counts per minute.
NY-ESO-1–specific CD4+ T cells are derived from distinct CD4+ T-cell populations showing different sensitivity to CD4+ CD25+ regulatory T cells between patients with and without NY-ESO-1 antibody. (A) CD4+CD25- T cells isolated from PBMCs were further separated into CD45RA+ or CD45RO+ using magnetic beads as described in “Study design.” (B) These T cells were cultured with APCs pulsed with appropriate NY-ESO-1 peptides according to respective HLA haplotype, namely NY-ESO-1157-170 for NC155, NY-ESO-187-98 for NC159, NY-ESO-1143-154 for NW681, NY-ESO-1121-132 for NW792, NY-ESO-187-98 for NW2010, and NY-ESO-1157-170 for NW2457, and tested for specific T-cell induction using cognate peptide or control HIV peptide by ELISPOT and proliferation assays. (C) Graded amounts of CD4+CD25+ T cells were added to cultures during in vitro peptide stimulation of NW681 and NW2010 and specific T-cell induction was examined using cognate peptides by ELISPOT and proliferation assays. These experiments were performed independently at least twice with similar results. Data are expressed as means +SD. CPM indicates counts per minute.
We have previously reported that CD4+ T-cell responses are correlated with NY-ESO-1 expression in tumor cells and NY-ESO-1 serum antibody. In this study, we have shown that NY-ESO-1–specific CD4+ T-cell precursors are derived from different populations in patients with or without NY-ESO-1 antibody and that differences in naturally occurring antitumor immunity are defined by distinctive CD4+CD25+ regulatory T-cell sensitivity of each population. It is still unknown if effector/memory T cells become resistant at a single-cell level or whether a relatively high number of precursors within memory population overwhelm suppression. A critical area for future exploration is to explain why some patients can induce memory NY-ESO-1–specific CD4+ T-cell responses even in the presence of naturally occurring CD4+CD25+ regulatory T cells. It is likely that these patients had an effective priming environment (eg, in which toll-like receptor signaling inhibits the generation/activation of CD4+CD25+ regulatory T cells)19,20 during the course of tumor development.
Although it has been shown that presence of CD4+CD25+ regulatory T cells at the local tumor site contributes to tumor growth and is associated with bad prognosis,14 there is no evidence for a correlation between human antigen-specific antitumor immunity and CD4+CD25+ regulatory T cells. Here, we have clearly shown that CD4+CD25+ regulatory T cells influence the induction of NY-ESO-1–specific antitumor immunity. It has been reported that CD4+CD25+ regulatory T cells require in vitro stimulation to gain their suppressive activity.6-8,21 It is possible that CD4+CD25+ regulatory T cells modulating NY-ESO-1 immunity are activated in an antigen-specific manner, comparable to the specific regulatory T cells generated against NY-ESO-1 gene family member LAGE-1 protein.22 Alternatively, some CD4+CD25+ regulatory T-cell populations that do not require further in vitro stimulation may exist and suppress induction of immunity.23,24
A unique finding in our study is that CD4+ T-cell responses to new NY-ESO-1 epitopes are induced from naive populations following CD4+CD25+ T-cell depletion, even in patients with preexisting immunity to NY-ESO-1. Our data provide an important hint for effective cancer vaccines during antigen priming through down-modulation of CD4+CD25+ regulatory T-cell function.
Prepublished online as Blood First Edition Paper, April 19, 2005; DOI 10.1182/blood-2005-02-0607.
Supported by the Cancer Vaccine Collaborative funded by the Cancer Research Institute and the Ludwig Institute for Cancer Research.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs E. Sato, T. Kato, and H. Shiku for helpful discussion, and E. Krapivinsky, C. Villalobos, and D. Santiago for technical support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal