Abstract
A crucial step in murine natural killer (NK) cell development, mediated by bone marrow stromal cells, is the induction of Ly49 and CD94/NKG2 receptor expression. The signals that regulate Ly49 receptor expression are still largely undetermined. It has been shown that interaction between lymphotoxin α1β2 (LTα1β2) and LTβ receptor (LTβR), expressed on lymphoid progenitor cells and nonlymphoid bone marrow stromal cells, respectively, is important for both quantitative and functional NK cell development. Therefore, we have investigated the role of LT-LTβR–mediated signaling in Ly49 and CD94/NKG2 receptor acquisition. We show that the NK receptor repertoire of LTβR–/– mice can only be partially analyzed because of the residual 129/Ola mouse genetic background, due to a physical linkage of the LTβR locus and the loci encoding the Ly49 and CD94/NKG2 receptors. Therefore, we transferred wild-type B6 lymphoid-committed progenitor cells into LTβR–/– mice, which differentiated into NK cells with a normal NK cell receptor repertoire. Also, administration of LTβR-immunoglobulin (Ig), which acts as a soluble receptor for LTα1β2, resulted in reduced NK cell percentages but did not influence the Ly49 and CD94/NKG2 receptor acquisition on remaining NK cells. These results indicate that LTβR-mediated signals are not required for Ly49 and CD94/NKG2 receptor acquisition.
Introduction
Lymphotoxin α (LTα), LTβ, and tumor necrosis factor α (TNFα) are structurally related cytokines belonging to the TNF ligand superfamily.1 In soluble forms, LTα and TNFα homotrimers interact with TNF receptor I and II, leading to a variety of inflammatory responses.2-4 LTα can also form a membrane-bound LTα1β2 heterotrimer that signals through the LTβ receptor (LTβR).3,4 LIGHT, another member of the TNF superfamily, has also been identified as a ligand for the LTβR.5
While LTα and LTβ are expressed in activated lymphocytes and natural killer (NK) cells,6 the expression of LTβR is restricted to nonlymphoid cells, including bone marrow (BM) stromal cells.7 Previous studies in mice deficient for LTα, LTβ, and LTβR have shown a profound role for LT-LTβR–mediated signaling in the secondary lymphoid organogenesis and function. Since lymph node development is not impaired in severe combined immunodeficient (SCID), double-negative recombination-activating gene 1 (RAG1–/–) and RAG2–/– mice, it has been assumed that NK progenitor cells, which express LTα1β2, are essential in secondary lymphoid formation.8-10 NK cells represent an important component of the innate immune system and are able to lyse a variety of virally infected cells and tumor cells without prior sensitization.11 Inhibitory and stimulatory receptors expressed on the surface of NK cells regulate NK cell cytotoxicity. In the mouse, the inhibitory receptors for major histocompatibility complex (MHC) class I molecules include the Ly49 and CD94/NKG2 receptor family (reviewed in Lanier12 and Raulet et al13 ). The signals inducing Ly49 receptor expression are poorly characterized, but BM stromal cells are indispensable for Ly49 receptor expression in vitro,14-16 and an intact BM microenvironment is required for complete NK cell maturation in vivo.17
Studies have shown that NK cell development and maturation are severely impaired in LTα–/– and LTβR–/– mice, with reduced NK cell percentages in spleen, BM, and blood.8,18-20 However, because of higher leukocyte numbers in spleen and blood,18,19 it is not always clear whether there was a similar reduction in the absolute NK cell number. Functionally, NK cells showed a reduced lytic capacity in vitro and an impaired antitumor function and recruitment of NK cells to lung and liver in vivo.19 It has been indicated that the defect in NK cell development may primarily be due to defective BM stromal cells.20 A model for the role of LT-LTβR–mediated signaling in NK cell differentiation in BM has been proposed: contact of membrane-bound LTα1β2-expressing hematopoietic progenitor cells with BM stromal cells activates the latter, which in turn induce interleukin 15 (IL-15) receptor expression on NK precursor cells. IL-15 is sufficient for the IL-15–responsive precursors to differentiate into immature NK1.1+ NK cells independent of BM stromal cells. Later on, BM stromal cells are, by an unidentified mechanism, required for the differentiation of immature NK1.1+ NK cells into Ly49 receptor expressing NK cells and for further functional maturation.20
Since NK cell development and lytic capacity is impaired in LTα–/– and LTβR–/– mice, signaling via the LTβR, which is expressed on BM stromal cells, seems to play an important role in NK cell differentiation. In addition, as the acquisition of Ly49 receptors, which is mediated by BM stromal cells, is a critical step in NK cell differentiation, we have addressed the role of LT-LTβR–mediated signals in the regulation of Ly49 and CD94/NKG2 receptor expression on NK cells. Using a combined analysis of B6 and LTβR–/– mice repopulated with lymphoid-committed progenitor cells and by administration of LTβR-IgG1 crystallizable fragment (Fc) fusion protein (LTβR-Ig), which acts as a soluble decoy receptor for LTα1β2 and LIGHT, we show that LT-LTβR interaction is not essential for the Ly49 and CD94/NKG2 receptor repertoire formation.
Materials and methods
Animals
C57BL/6J (B6) and 129/Ola mice were originally purchased from Harlan Netherlands (Zeist, The Netherlands). LTβR–/– mice (N5 backcrossed to C57BL/6) were obtained from the Institute of Medical Microbiology, Immunology, and Hygiene of the Technical University of Munich, Munich, Germany. Congenic C57BL/6-Ly5.1 (B6-CD45.1) mice were originally purchased from Charles River Laboratories France (Les Oncins, France). 129/Sv mice were kindly provided by Dr P. Brouckaert (Ghent University, Ghent, Belgium) and by Dr P. Matthys (Catholic University, Leuven, Belgium). Mice were housed and bred under specific pathogen-free conditions in our breeding facility and were treated and used in agreement with the guidelines of the local ethical committee.
Antibodies and soluble receptor fusion protein
Monoclonal antibodies (mAbs) used for labeling were anti-NK1.1 (fluorescein isothiocyanate [FITC]– and phycoerythrin [PE]–conjugated, clone PK136), anti-CD49b (FITC- and PE-conjugated, clone DX5), anti-CD3 (allophycocyanin [APC]– and peridinin chlorophyll protein [PerCP]–conjugated, clone 145-2C11), anti–c-kit (CD117) (PE-conjugated, clone 2B8), anti-Ly49C/I (PE-conjugated, clone 5E6), and anti-Ly49D (FITC-conjugated, clone 4E5), all obtained from Pharmingen (San Diego, CA), and anti-CD122 (IL-2Rβ) (FITC- and biotin-conjugated, clone TM-β1; kindly provided by Dr T. Tanaka, Tokyo, Japan), anti-Ly49E/C (FITC-conjugated, clone 4D12),21 anti-Ly49A (FITC-conjugated, clone JR9-318; kindly provided by Dr J. Roland, Institut Pasteur, Paris, France), anti-Ly49A/D (biotin-conjugated, clone 12A8; kindly provided by Dr J. R. Ortaldo, National Cancer Institute, Frederick, MD), anti-Ly49H (FITC-conjugated, clone 3D10; provided by Dr W. Yokoyama, Howard Hughes Medical Institute, St Louis, MO), anti-Ly49G2 (FITC-conjugated, clone 4D11), obtained from American Type Culture Collection (Rockville, MD), anti-NKG2A/C/E (FITC-conjugated, clone 3S9),21 anti-Sca2 (FITC-conjugated, clone MTS-35; kindly provided by Dr R. Boyd, Monash University Medical School, Australia), anti-FcγRII/III (unconjugated, clone 2.4G2; kindly provided by Dr J. Unkeless, Mount Sinai School of Medicine, New York, NY). Biotin-conjugated monoclonal antibodies used for lineage depletion were anti-B220 (clone RA3-3A1; American Type Culture Collection), anti-CD11b (clone M1/70), anti-CD2 (clone 12.15A, kindly provided by Dr B. Kyewski, FSP Tumor Immunology Programme, Heidelberg, Germany), anti-Gr1 (clone Rb6-8C5; kindly provided by Dr B. Fazekas de St. Groth, Centenary Institute of Cell Biology and Cancer Medicine, Sydney, Australia), and anti-TER119 (clone TER119; kindly provided by Dr B. Fazekas de St. Groth). Second-step reagentia used were streptavidin-APC and -PE (Becton Dickinson, Mountain View, CA) and streptavidin Tri-color conjugate (Caltag Laboratories, Burlingame, CA). Murine LTβR-Ig and control human IgG were kindly provided by Dr J. Browning (Biogenidec, Cambridge, MA).
Preparation of cell suspensions
Spleens from 8- to 12-week-old B6, LTβR–/–, 129/Ola, and 129/Sv mice were removed and disrupted. Adult bone marrow cells were isolated from 8- to 12-week-old B6-CD45.1 mice by irrigation of the femurs and tibias. Erythrocytes from spleens and bone marrow were lysed with 0.17 M NH4Cl.
Sorting and flow cytometric analysis
Adult bone marrow from B6-CD45.1 mice were depleted from lineage-positive (Lin+) cells, and c-Kit+Sca2+Lin– cells were sorted as described before.22 Splenocytes were enriched with Anti-NK Cell (DX5) Microbeads (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions, and incubated with combinations of mAbs. NK cells from spleens of LTβR-Ig–treated and control human IgG–treated mice were enriched by negative selection using anti-CD4, anti-CD8, and anti-IgG beads (Dynal, Hamburg, Germany) according to manufacturer's instructions. The FcγR was blocked by preincubation of cells with saturating amounts of anti-FcγRII/III mAb to avoid aspecific binding. The cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) with the CellQuest software program for data acquisition and analysis.
In vivo repopulation studies
Sorted c-kit+Sca2+Lin– cells (70 000-100 000) from adult B6-CD45.1 mouse bone marrow were injected intravenously into B6 and LTβR–/– mice that were irradiated (900 cGy) 24 hours prior to injection. Four to 5 weeks after injection, flow cytometric analysis was performed on DX5-enriched splenocytes.
RT-PCR
TRIzol (Life Technologies, Gaithersburg, MD) was added to isolated DX5+ splenocytes, and RNA was extracted according to the manufacturer's instructions. Before reverse transcription (RT), digestion of DNA was performed with deoxyribonuclease I (Life Technologies). cDNA was synthesized with oligo(dT) as primer using the Superscript kit (Life Technologies). For hypoxanthine phosphoribosyltransferase (HPRT), a housekeeping enzyme, oligonucleotides were GTA ATG ATC AGT CAA CGG GGG AC (sense primer) and CCA GCA AGC TTG CAA CCT TAA CCA (reverse primer). The polymerase chain reaction (PCR) profile and the oligonucleotides used for amplification of the different members of the Ly49 gene family from B6 mice were previously described.23 The oligonucleotides used for PCR amplification of the 129/Sv Ly49 gene family members were CGC CAT GTT ATT ACA GAG GG (sense primer) and GTC CTC TCT CAA CAG GAT AG (reverse primer) for Ly49U/I, TTC CCT CAT TGA CTC TCT GAC A (sense primer) and GGG GAC CAG AGA AGA TCT GTT T (reverse primer) for Ly49O, and GGA TTG ACA ATC ACC CAT CTA AG (sense primer) and GAG AAC ATT CCA AAA ATC TTC AG (reverse primers) for Ly49V. PCR amplification was performed using a PTC-200 DNA Engine (MJ Research, Waltham, MA) with 30 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute (HPRT), and with 40 cycles of 94°C for 30 seconds, 51°C for 30 seconds, and 72°C for 1 minute for the different Ly49 genes from 129/Sv mice. The amount of template used for PCR amplification of the different Ly49 genes was normalized to HPRT expression.
Results
Distinct Ly49 receptor repertoire in LTβR–/– mice
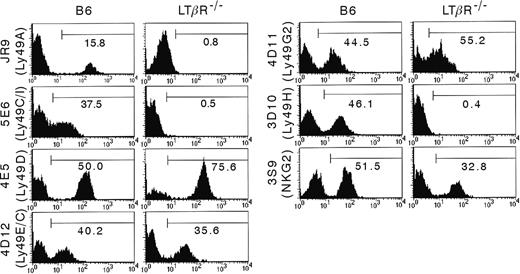
It has been shown that mice with disrupted LT-LTβR interaction are characterized by both quantitative and functional NK cell defects.8,20 Also in the LTβR–/– mice we examined, a reduced cytotoxic activity of the NK cells was observed (data not shown). Here, we examined a possible role of LTβR in the formation of the NKG2/CD94 and Ly49 receptor repertoire on NK cells. DX5+CD122+CD3– gated NK cells from splenocyte suspensions of B6 and LTβR–/– mice were analyzed by flow cytometry (Figure 1). When comparing the cell surface expression of a panel of the Ly49 receptors and of CD94/NKG2, some striking differences between B6 and LTβR–/– mice were observed: Ly49A+, Ly49C/I+, Ly49H+, and CD94/NKG2lo NK cells were completely absent in LTβR–/– mice, and the percentage of CD94/NKG2hi cells was decreased. However, the percentage of Ly49D+ and Ly49G2+ NK cells was increased. To further investigate whether the different protein expression pattern was reflected at the mRNA level, RT-PCR was performed on isolated RNA from DX5-enriched splenocytes. As shown in Figure 2, and in agreement with protein expression, there was a strong reduction in the mRNA expression level in LTβR–/– NK cells for ly49a and ly49h, while transcripts for ly49c could not be detected. The ly49g2 mRNA levels were comparable (data not shown). Surprisingly, and in contrast to the surface expression of Ly49D, no ly49d transcripts were present in LTβR–/– mice.
Distinct Ly49 and CD94/NKG2 cell surface expression by B6 versus LTβR–/–NK cells. Splenocytes were isolated from B6 and LTβR–/– mice and were stained with mAbs for DX5, CD122, CD3 and for the indicated Ly49 receptors and CD94/NKG2. Analysis was performed on DX5+CD122+CD3– gated splenocytes. The results are presented as the percentage of positive cells in the region indicated by the horizontal bar, and are representative for 4 experiments.
Distinct Ly49 and CD94/NKG2 cell surface expression by B6 versus LTβR–/–NK cells. Splenocytes were isolated from B6 and LTβR–/– mice and were stained with mAbs for DX5, CD122, CD3 and for the indicated Ly49 receptors and CD94/NKG2. Analysis was performed on DX5+CD122+CD3– gated splenocytes. The results are presented as the percentage of positive cells in the region indicated by the horizontal bar, and are representative for 4 experiments.
The residual 129/Ola genetic background determines the Ly49 receptor expression pattern of LTβR–/– NK cells
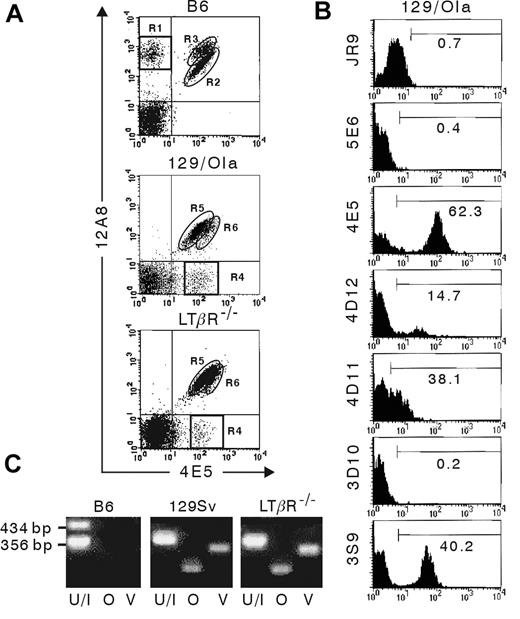
LTβR–/– mice, originally generated using E14.1 embryonic stem (ES) cells, which are of 129/Ola origin, were backcrossed 5 times onto the B6 strain.24 However, it has been shown that LTβR–/– mice have no NK1.1 surface expression because of a physical linkage of the NK1.1 locus to the Ltbr gene.20 Indeed, on mouse chromosome 6, the Ltbr gene is only separated by 1.7 centiMorgan (cM) from the Klrb1c gene, encoding the NK1.1 receptor. Importantly, also the genes encoding the different Ly49 receptors are located in the same region on chromosome 6 and are separated by 2.22 cM (ly49e) to 2.32 cM (ly49a) from the Ltbr gene. Consequently, upon each backcross there is an approximate 97% probability that the LTβR locus and the Ly49 receptor loci are not separated by recombination. To determine the role of the genetic background on the Ly49 receptor expression, flow cytometric analysis was performed using the 4E5 and 12A8 mAbs. Previously, it has been shown that staining of B6 and 129/J NK cells with these mAbs generates a different expression pattern with 4 distinct populations25,26 : a 4E5–12A8+ population (Figure 3A, R1), corresponding to Ly49AB6-expressing NK cells, is exclusively present in B6 mice. The 4E5+12A8lo NK cells (Figure 3A, R2) express only Ly49DB6, and the 4E5+12A8hi population (Figure 3A, R3) coexpresses Ly49AB6 and Ly49DB6. Conversely, a 4E5+12A8– population (Figure 3A, R4), which represents Ly49O129/J- or Ly49V129/J-expressing NK cells, is exclusively present in 129/J mice. The 4E5lo12A8+ population (Figure 3A, R5) represents the Ly49R129/J-expressing NK cells. The 4E5hi12A8+ (Figure 3A, R6) NK cells coexpress Ly49R129/J and Ly49O129/J and/or Ly49V129/J. In Figure 3A it is shown that 4E5+12A8– and 4E5hi12A8+ NK cells are present in LTβR–/– mice, whereas 4E5–12A8+ and 4E5+12A8hi NK cells are absent. This result indicates that at least some Ly49 members of LTβR–/– NK cells are of the 129/Ola genetic background. This is in agreement with receptor repertoire analysis of NK cells from 129/Ola mice. Figure 3B shows that the antibodies JR9-318, 5E6, and 3D10, recognizing Ly49A, Ly49C/I, and Ly49H, respectively, in the B6 background, do not stain NK cells from 129/Ola mice. The 3S9 antibody, recognizing NKG2, stains a subpopulation of the 129/Ola NK cells very brightly, but the remaining NK cells are not weakly stained, as is the case for B6 NK cells. Instead, this fraction was found to be entirely negative for staining with 3S9. There are some differences between LTβR–/– (Figure 1) and 129/Ola mice (Figure 3B) in the percentages of NK cells stained with mAbs 4E5+, 4D12+, and 4D11+, which recognize Ly49D, Ly49E/C, and Ly49G2, respectively, in the B6 background, and which are higher in LTβR–/– mice, and in the 3S9hi (NKG2hi) NK percentage, which is lower in LTβR–/– mice compared with 129/Ola mice. These differences of LTβR–/– versus 129/Ola NK cells are minor for 4E5 (74.7% ± 3.2% versus 65.8% ± 4.7%; P < .05) and 3S9hi (32.4% ± 4.5% versus 40.8% ± 3.2%; P < .05), but larger for 4D12 (34% ± 1.8% versus 12.4% ± 3.7%; P < .01) and 4D11 (53.3% ± 3% versus 36.3% ± 3.8%; P < .01).
Differences in Ly49 mRNA levels between B6 and LTβR–/–mice. RNA was extracted from freshly isolated and DX5-enriched splenocytes from B6 and LTβR–/– mice. Semiquantitative RT-PCR was performed with primers generated for Ly49 receptor family members ly49a, ly49c, ly49d, and ly49h from B6 mice.
Differences in Ly49 mRNA levels between B6 and LTβR–/–mice. RNA was extracted from freshly isolated and DX5-enriched splenocytes from B6 and LTβR–/– mice. Semiquantitative RT-PCR was performed with primers generated for Ly49 receptor family members ly49a, ly49c, ly49d, and ly49h from B6 mice.
Expression of 129/Ola alleles of Ly49 receptors on LTβR–/–NK cells. Flow cytometric analysis (A-B) was performed on freshly isolated splenocytes from B6, 129/Ola, and LTβR–/– mice. (A) DX5-enriched cells were stained with mAbs for DX5, CD3 and with 12A8 and 4E5 mAbs. The regions R1 to R6, which are referred to in the text, are indicated. (B) Splenocytes from 129/Ola mice were stained with mAbs for DX5, CD122, and CD3 and with the indicated antibodies specific for the different Ly49 receptors and CD94/NKG2. The results are presented as the percentage of positive cells in the region indicated by the horizontal bar. Analysis was performed by gating on (A) DX5+CD3– splenocytes or (B) DX5+CD122+CD3– splenocytes. (C) RNA was extracted from DX5-enriched splenocytes from the indicated mouse strains, and RT-PCR was performed using primers generated for Ly49 receptor family members ly49u/i (U/I), ly49o (O), and ly49v (V) from 129/J mice. The length of the PCR products obtained with Ly49U/I primers are indicated.
Expression of 129/Ola alleles of Ly49 receptors on LTβR–/–NK cells. Flow cytometric analysis (A-B) was performed on freshly isolated splenocytes from B6, 129/Ola, and LTβR–/– mice. (A) DX5-enriched cells were stained with mAbs for DX5, CD3 and with 12A8 and 4E5 mAbs. The regions R1 to R6, which are referred to in the text, are indicated. (B) Splenocytes from 129/Ola mice were stained with mAbs for DX5, CD122, and CD3 and with the indicated antibodies specific for the different Ly49 receptors and CD94/NKG2. The results are presented as the percentage of positive cells in the region indicated by the horizontal bar. Analysis was performed by gating on (A) DX5+CD3– splenocytes or (B) DX5+CD122+CD3– splenocytes. (C) RNA was extracted from DX5-enriched splenocytes from the indicated mouse strains, and RT-PCR was performed using primers generated for Ly49 receptor family members ly49u/i (U/I), ly49o (O), and ly49v (V) from 129/J mice. The length of the PCR products obtained with Ly49U/I primers are indicated.
Additional data were obtained by RT-PCR using primers for Ly49 members of 129/J mice (Figure 3C). With primers specific for the genes encoding Ly49O129/J and Ly49V129/J, which show highest amino acid similarity to Ly49AB6, a fragment of the correct length was generated starting from mRNA isolated from DX5-enriched splenocytes from LTβR–/– and 129/Sv mice, but no PCR product was obtained from B6 mice. Using primers for Ly49u/i129/J, a specific fragment (356 base pair [bp]) was generated from LTβR–/– and 129/Sv mRNA, while 2 bands were generated from B6 mRNA corresponding to ly49iB6 and/or ly49hB6 (∼ 355 bp) and ly49cB6 (434 bp). It has been shown that the ly49v129 and ly49o129 genes are at both ends in the natural killer gene complex, except for the Ly49Q and ly49e genes which are more centromeric, and closer to the Ltbr gene than ly49v.27 On the basis of this report and on our results, it can be assumed that presumably most, if not all, of the ly49 genes in LTβR–/– NK cells are of 129 origin.
Acquisition of a normal Ly49 repertoire upon transfer of WT hematopoietic stem cells into LTβR–/– mice
To investigate the role of the LT-LTβR interaction on the NK receptor formation without the influence of the 129/Ola genetic background, c-kit+Sca2+Lin– lymphoid-committed progenitor cells were sorted from congenic B6-CD45.1 mice and injected intravenously in irradiated (900 cGy) B6 and LTβR–/– mice, which both express the CD45.2 allele. The transfer of c-kit+Sca2+Lin– lymphoid-committed progenitor cells from congenic B6-CD45.1 mice into B6 and LTβR–/– mice clearly overcomes the problems that are inherent to the presence of Ly49129/Ola receptors in LTβR–/– mice with a B6 background and which are addressed further in “Discussion.” Since in the transfer experiments both B6 and LTβR–/– mice were injected with donor cells from the same congenic mouse strain, the sole difference between both acceptor strains is expression of LTβR. Another important advantage of these transfer studies is that a larger panel of Ly49 receptors can be addressed by using mAbs with clearly determined specificity for Ly49 and CD94/NKG2 receptors from B6 mice. The main reason for transferring lymphoid-restricted hematopoietic precursor cells was to have clean experimental conditions in which the progeny of the injected cells were only lymphoid cells. This is particularly relevant, as reconstitution with “contaminating” LTβR-expressing cells would profoundly influence the results in LTβR-deficient recipients. This could indeed be the case when total bone marrow cells are injected, which contain stromal cells or progenitors thereof. A consequence of the requirement to use lymphoid-restricted hematopoietic precursor cells is that we could not assess the long-term peripheral NK cell pool.
Four to 5 weeks after injection, flow cytometric analysis was performed on gated CD45.1 NK1.1+ CD3– splenic NK cells. Analysis of the surface expression of a panel of Ly49 receptors and of CD94/NKG2 is shown in Figure 4. While for most NK cell receptors surface expression was comparable, Ly49A and CD94/NKG2 expression was significantly lower (P < .01). It should be noted, however, that the difference in Ly49A expression was very small (14.39% ± 0.79% versus 10.68% ± 1.44% in wild-type [WT] and LTβR–/– recipients, respectively). Acquisition of the different Ly49 members occurs in an orderly and time-dependent fashion, where Ly49A is the last receptor to be expressed, and where CD94/NKG2 expression is high at early time points and decreases thereafter.22 Therefore, small differences in the rate of NK cell differentiation between both strains could explain the minor differences in Ly49A receptor and CD94/NKG2 receptor repertoire.
Blocking of the LT-LTβR interaction does not significantly alter the Ly49 receptor repertoire
In a second approach to determine a possible role for LTβR-mediated signaling on the Ly49 receptor repertoire, we blocked the LT-LTβR interaction via soluble LTβR-Ig. Previous studies have shown that administration of LTβR-Ig after birth generates a phenotype with a disorganized splenic architecture28 and shows that continuous signaling through the LTβR is necessary to maintain NK cell homeostasis in adult mice.20
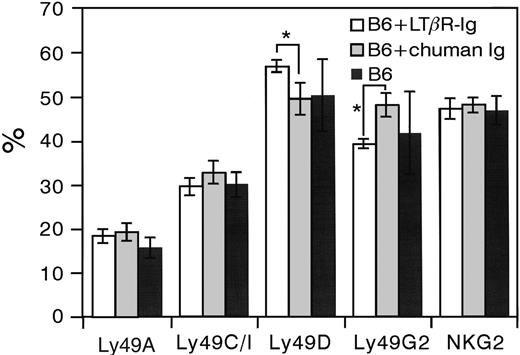
B6 mice were injected once a week during 5 weeks with 100 μg LTβR-Ig or control human Ig. LTβR-Ig treatment resulted, as expected,28 in the loss of splenic architecture (data not shown). Similar to results from Wu et al,20 NK percentages in spleen were reduced by 40% to 50% after administration of LTβR-Ig but not of the control human Ig (data not shown). Results of flow cytometric analysis of a panel of Ly49 receptors and CD94/NKG2 on NK cells are shown in Figure 5. Blocking of the LT-LTβR interaction did not seem to influence Ly49A, Ly49C/I, or CD94/NKG2 expression. Ly49D and Ly49G2 expression on LTβR-Ig–treated B6 mice was significantly higher (P < .05) and lower (P < .01), respectively, compared with B6 mice injected with control human Ig. However, the differences were rather small, and it should also be noted that Ly49D and Ly49G2 expression on LTβR-Ig–treated B6 mice was not significantly different from normal B6 mice. Therefore, it can be assumed that the role of LTβR blocking on Ly49D and Ly49G2 expression is negligible.
Ly49 and CD94/NKG2 receptor expression on NK cells after transfer of wild-type lymphoid progenitor cells into LTβR–/–mice. Sorted lymphoid-committed progenitor cells from congenic B6-CD45.1 mice were injected intravenously in irradiated B6 (n = 3) and LTβR–/– (n = 4) mice, which both express the CD45.2 allele. Four to 5 weeks after injection flow cytometric analysis was performed. DX5-enriched splenocytes from both strains were stained with mAbs for NK1.1, CD3, CD45.1, and the indicated Ly49 receptors or CD94/NKG2. Expression percentages of the indicated Ly49 receptors and CD94/NKG2 were obtained by gating on CD45.1+NK1.1+CD3– NK cells. *P < .05 by Student t test.
Ly49 and CD94/NKG2 receptor expression on NK cells after transfer of wild-type lymphoid progenitor cells into LTβR–/–mice. Sorted lymphoid-committed progenitor cells from congenic B6-CD45.1 mice were injected intravenously in irradiated B6 (n = 3) and LTβR–/– (n = 4) mice, which both express the CD45.2 allele. Four to 5 weeks after injection flow cytometric analysis was performed. DX5-enriched splenocytes from both strains were stained with mAbs for NK1.1, CD3, CD45.1, and the indicated Ly49 receptors or CD94/NKG2. Expression percentages of the indicated Ly49 receptors and CD94/NKG2 were obtained by gating on CD45.1+NK1.1+CD3– NK cells. *P < .05 by Student t test.
Ly49 and CD94/NKG2 receptor expression on NK cells following administration of neutralizing LTβR-Ig. B6 mice were injected once a week during 5 weeks with 100 μg LTβR-Ig or control human Ig (chuman Ig). One week after the last injection, flow cytometric analysis was performed on splenic NK cells enriched by negative selection using anti-CD4, anti-CD8, and anti-IgG beads. Cells were incubated with a combination of mAbs for NK1.1, CD3, and the indicated Ly49 receptors or CD94/NKG2. Data were collected from gated NK1.1+CD3– NK cells and are presented as the mean ± SD. *Statistically significant differences (by Student t test) between LTβR-Ig (n = 3) and chuman Ig (n = 5) treated mice. Results from B6 control stainings (n = 8) are also shown.
Ly49 and CD94/NKG2 receptor expression on NK cells following administration of neutralizing LTβR-Ig. B6 mice were injected once a week during 5 weeks with 100 μg LTβR-Ig or control human Ig (chuman Ig). One week after the last injection, flow cytometric analysis was performed on splenic NK cells enriched by negative selection using anti-CD4, anti-CD8, and anti-IgG beads. Cells were incubated with a combination of mAbs for NK1.1, CD3, and the indicated Ly49 receptors or CD94/NKG2. Data were collected from gated NK1.1+CD3– NK cells and are presented as the mean ± SD. *Statistically significant differences (by Student t test) between LTβR-Ig (n = 3) and chuman Ig (n = 5) treated mice. Results from B6 control stainings (n = 8) are also shown.
Discussion
Studies in recent years have shown that the different Ly49 receptors are expressed in an ordered and successive manner on developing NK cells and that the accumulation is terminated when the NK cell expresses receptors with sufficient avidity for self-MHC class I molecules.14-16,22,29,30 However, little is known about actual molecular signals that induce the expression and control the acquisition of Ly49 and CD94/NKG2 receptors. Recent studies on mice deficient for the transcription factors T-cell factor-1 (TCF-1), PU.1, and GATA3 indicated a role for these transcription factors in the modulation of expression of some Ly49 receptors.31-35
In the past, the role of IL-15 in NK cell function, development, and homeostasis has extensively been studied.36-40 In IL-15–deficient mice, and in mice deficient for any of the IL-15 receptor (IL-15R) subunits, such as IL-15Rα,41,42 IL-2/15Rβ,43 or γc,44 NK cell development is severely impaired. A role for IL-15/IL-15Rα mediated signaling in Ly49 receptor induction has been demonstrated, as a strong reduction in Ly49 receptor expression by NK cells is observed in mice deficient for IL-15Rα or IL-15. However, Ly49 expression is not completely absent,42 indicating a crucial role for other regulatory factors.
Wu et al20 have proposed a model for the role of LT-LTβR signaling in early stages of NK cell development: LT-expressing cells, like early NK cell precursors, are thought to activate LTβR-expressing stromal cells, which in turn induce IL-15R expression on NK precursors and subsequent presentation of IL-15 is sufficient to drive the NK precursors to NK1.1 expression. However, in vitro culture of LTα–/– BM cells with exogenously added IL-15, but in the absence of stromal cells, generates equal NK cells numbers as cultured WT BM cells, showing that IL-15 alone can overcome the arrest in NK cell development of LTα–/– BM cells.8 Consequently, it can be suggested that LT-LTβR interaction controls a developmental checkpoint independent of IL-15/IL-15R function. A second possible explanation could be that the small numbers of NK precursors that are generated in LTα–/– mice are selectively expanded upon administration of IL-15 in vitro.
A critical step in later stages of NK cell differentiation and maturation is the induction of Ly49 receptor expression, which is absolutely dependent on a cell-to-cell contact with an intact BM microenvironment.17 It has been suggested that the impaired NK cell development in LTα–/– and LTβR–/– mice is mainly due to defective stromal cells.20 As suggested by Wu et al,20 LT-LTβR interaction activates BM stromal cells. Possibly, this could result in the expression of unknown molecules playing a role in Ly49 receptor induction.
Analysis of the NK receptor repertoire of B6 versus LTβR–/– mice showed clear differences (Figure 1). However, our results on the double-labeling with 4E5 and 12A8 antibodies (Figure 3A) and on the RT-PCR using Ly49129/Sv-specific primers (Figure 3C), combined with the results from a previous study,27 showed that presumably most, if not all, of the Ly49 receptors of LTβR–/– mice are of 129/Ola origin. Therefore, we compared the Ly49 expression on NK cells from LTβR–/– (Figure 1) versus 129/Ola mice (Figure 3B). This comparison showed some differences, which were either minor (4E5 staining) or larger (4D11 and 4D12 stainings). To explain these differences, it can be hypothesized that they are the result of LTβR deficiency. If this is the case, LTβR expression is not required for Ly49 expression as in the stainings in which we noticed a difference in expression, the expression of Ly49 members was higher on the LTβR–/– NK cells. A second hypothesis is that the expression of the panel of Ly49 and CD94/NKG2 receptors from 129/Ola origin in LTβR–/– mice is influenced by their B6 background, as the LTβR–/– mice have been backcrossed 5 times on a B6 background.24 Notwithstanding the same MHC class I expression (H-2b), other factors can indeed influence the expression level of NK receptors. It has been shown that the Ly49 receptor repertoire formation can be influenced by, among other factors, the transcription factor TCF-132,33 and by the src family kinase Fyn.45 Small differences in the expression level of these factors between B6 and 129/Ola mice could result in a somewhat different receptor repertoire. To overcome the problems inherent to the presence of Ly49129/Ola receptors in LTβR–/– mice with a B6 background, we transferred c-kit+Sca2+Lin– lymphoid-committed progenitor cells from congenic B6 CD45.1 mice into B6 and LTβR–/– mice. Our results show no influence of the deficient LT-LTβR signaling on Ly49 receptor expression by the differentiated NK cells (Figure 4), and they provide evidence to reject the first hypothesis. In addition, our results obtained by neutralization of LTα1β2 by LTβR-Ig show no change in Ly49 receptor induction or expression regulation (Figure 5). Hence, the assumption of an unknown receptor for membrane-bound LTα1β2 that plays a role in NK receptor repertoire formation is unlikely. As expected,28,46 administration of LTβR-Ig resulted in our experiments in the loss of splenic organization (data not shown), indicating that the LTβR-Ig treatment was functional. A possible explanation for the fact that LT-LTβR interaction blocking had no influence on Ly49 receptor expression by splenic NK cells could be that blocking only influences Ly49 and CD94/NKG2 receptor expression on newly generated NK cells and that turnover of NK cells is too slow. However, similar to previous results by Wu et al,20 the prolonged treatment for 5 weeks with LTβR-Ig resulted in a 46% reduction in NK1.1+ CD3– NK cell percentages in spleen, in agreement with the assumption that continuous signaling through the LTβR is required for NK cell homeostasis. It has also been shown that the life span of nonproliferating NK cells is about 10 days and that upon transfer of NK cells into NK-deficient mice the donor-derived NK cell numbers decrease with a half-life of around 10 days.39 Therefore, it can be assumed that the majority of splenic NK cells in mice treated with LTβR-Ig for 5 weeks were newly generated.
While this manuscript was in preparation, results from a study of Lian et al47 were published, in which the role of LT in the acquisition of Ly49 receptors during NK cell development was addressed. It was shown that in LTα–/– mice expression percentages of all Ly49 receptors examined on splenic NK cells is reduced to approximately 50% of normal WT expression. Surprisingly, they also showed that expression of some Ly49 members is less affected (Ly49C/I/G2) or even increased (Ly49D/H) on BM NK cells of LTα–/– mice. In addition, in LTα transgenic (LTαTg) mice, where NK cell numbers are significantly higher, there seems to be no or only a minor effect on Ly49 receptor expression. Similar to our studies, LT-LTβR interaction blocking was performed with LTβR-Ig, but, contrary to our results where no influence could be demonstrated, they observed a significant reduction in Ly49 receptor expression. It should, however, be noted that Lian et al47 observed no or only a minor reduction in splenic NK cell percentages after LTβR-Ig administration, in contrast to a previous study by Wu et al20 and to our results, in which approximately 50% reduction was obtained. A possible explanation for the difference in results between the 2 studies could be the difference in the LTβR-Ig treatment: intraperitoneal administrations of 100 μg LTβR-Ig every 3 days for 3 or 5 doses47 compared with 1 administration per week of 100 μg LTβR-Ig for 5 weeks in our studies. It should also be noted that WT mice were injected with phosphate-buffered saline, and not with control human Ig as in our studies, so the effect of the LTβR-Ig administration could still be aspecific. A key experiment, which was not performed by Lian et al47 but which could have provided interesting data on the role of LT-LTβR interaction, is administration of agonistic anti-LTβR mAb in LTα–/– mice. However, since it has been shown that transfer of WT BM cells in LTα–/– mice cannot restore splenic NK percentages,8 while splenic architecture is restored,48 it is possible that administration of the agonistic mAb cannot correct potential developmentally fixed changes in LTα–/– BM environment.
Our data show that the role of LT-LTβR–mediated signaling on the NK cell repertoire in LTβR-deficient mice can only be partially addressed due to the physical linkage of the NK receptors to the LTβR locus. Since the LTα gene is not linked to chromosome 6, it could be assumed that the influence of LTα deficiency on the Ly49 receptor repertoire is not related to the presence of 129/Ola-linked genes. While we have no clear-cut explanation for the different NK cell receptor repertoires in LTα–/– and LTβR–/– mice, we suggest that LTα deficiency potentially leads to additional effects. LTα can either be present as a homotrimer, which binds to TNFRI/II and herpesvirus entry mediator (HVEM).5
A final remark, already mentioned by other studies, is that particular attention should be paid when NK-related functions are studied in gene-targeted mice generated using 129-derived embryonic stem cells. In addition to the expression of different Ly49 alleles, it has been shown that in 129/J and 129/Sv mice cross-linking of DNAX activation protein 12 (DAP12)–associated receptors, like Ly49D, results in DAP12 phosphorylation, but not in calcium mobilization or cytokine production.26 An efficient and fast way to determine a possible interfering genetic background is flow cytometric analysis using the 4E5 and 12A8 mAbs, which stain distinct NK cell populations in B6 and 129 mice25,26 (and this manuscript).
Prepublished online as Blood First Edition Paper, April 12, 2005; DOI 10.1182/blood-2004-10-4159.
Supported by grants from the Fund for Scientific Research-Flanders (Belgium), the Research-fund of Ghent University (F.S. and K.V.B.), and the Belgian Federation against Cancer, nonprofit organization.
F.S. and K.V.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr J. Roland, Dr J. R. Ortaldo, Dr W. Yokoyama, Dr R. Boyd, Dr J. Unkeless, Dr B. Kyewski, and Dr B. Fazekas for providing mAbs; Dr J. Browning for providing murine LTβR-Ig and control human Ig; and Dr P. Brouckaert and Dr P. Matthys for providing 129/Sv mice. K. Pfeffer thanks the Deutsche Forschungsgemeinschaft (DFG) for support. We also thank M. De Smedt for technical advice, C. Collier and G. De Smet for animal care, P. Dewint for help with injections, C. De Boever for art work, and Dr E. Veys for his continuous support. V.D.C. and A.S.F. are research assistants of the Fund for Scientific Research-Flanders. F.S. is a PhD student and K.V.B. is a postdoctoral student.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal