Comment on Condliffe et al, page 1432
Signaling enzymes of the phosphoinositide 3-kinase (PI3K) family are essential for neutrophil respiratory burst. Condliffe and colleagues have made use of novel selective PI3K inhibitors and mutant mouse strains to illuminate the roles of distinct PI3K subtypes in this process.
Production of reactive oxygen species (ROS) by activated neutrophils contributes to pathogen elimination by direct microbicidal effects and by indirect effects on the activated neutrophil and local tissue. ROS production, also called respiratory burst, is triggered by neutrophil cell surface receptors that recognize bacterial components (eg, N-formyl-methyonyl-leucyl-phenylalanine [fMLP]) or host inflammatory molecules (eg, the complement cascade [C5a]). An important mechanism that limits ROS production specifically to sites of infection is the “priming” of neutrophils, whereby initial exposure to inflammatory cytokines such as tumor necrosis factor-α (TNF-α) greatly augments the subsequent respiratory burst triggered by agonists like fMLP and C5a.
PI3K is a key signaling intermediate linking various neutrophil surface receptors to the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex that generates ROS (see figure).1 PI3K enzymes generate 3-phosphorylated inositol lipids that direct the assembly of membrane-associated signaling complexes. Two of these lipid products, phosphatidylinositol (PtdIns)-3,4,5-trisphosphate (PIP3) and PtdIns-3,4-bisphosphate, are triggered by fMLP and C5a prior to and during the respiratory burst. Broad spectrum PI3K inhibitors such as wortmannin inhibit respiratory burst, and neutrophil priming is associated with enhanced PIP3 production following agonist treatment.
PI3K enzymes in mammals are a family of 8 catalytic isoforms, divided into 4 subgroups based on sequence homology, substrate selectivity, and regulation; all are expressed in leukocytes.2 Wortmannin and another commonly used PI3K inhibitor, LY294002, inhibit most of these isoforms and have additional effects on various other cellular enzymes. This not only prevents their use as therapeutic drugs, but also has necessitated the development of more sophisticated approaches to chart the details of PI3K signaling specificity in the respiratory burst and other cellular processes.3 An important advance was the demonstration in 2000 by 3 groups that deletion of the gene encoding the class IB PI3K p110γ in mice nearly abolishes agonist-mediated PIP3 production and ROS generation in phagocytes (reviewed in Dekker and Segal4 ). The current paper by Condliffe and colleagues uses recently developed selective PI3K inhibitors to probe the details of PI3K signaling, and uncovers some surprising differences between mouse and human neutrophils.FIG1
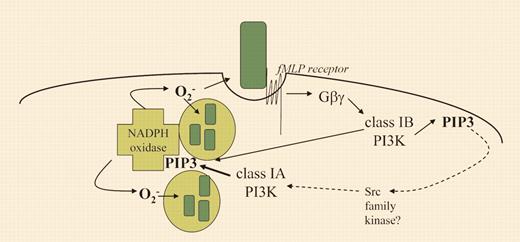
Agonist-driven ROS production in primed human neutrophils involves sequential activation of 2 PI3K subtypes. This figure depicts bacteria (green rectangles) being recognized by the cell surface fMLP receptor, triggering phagocytosis and activation of the NADPH oxidase complex. Within 10 seconds of fMLP binding, PIP3 levels rise via the G-protein–dependent activation of class IB PI3K (p110γ). A second peak of PIP3 is observed around 1 to 2 minutes, coinciding with the peak levels of superoxide production. This second peak is largely dependent on class IA PI3K (thick arrow), but absolutely requires prior activation of the class IB PI3K. The steps leading from the initial PIP3 generation to class IA PI3K activation are not established (dashed lines). However, the Src family kinase inhibitor PP1 attenuates the second peak of PIP3, implicating this kinase family in the pathway. PIP3 and other PI3K products promote NADPH oxidase function.
Agonist-driven ROS production in primed human neutrophils involves sequential activation of 2 PI3K subtypes. This figure depicts bacteria (green rectangles) being recognized by the cell surface fMLP receptor, triggering phagocytosis and activation of the NADPH oxidase complex. Within 10 seconds of fMLP binding, PIP3 levels rise via the G-protein–dependent activation of class IB PI3K (p110γ). A second peak of PIP3 is observed around 1 to 2 minutes, coinciding with the peak levels of superoxide production. This second peak is largely dependent on class IA PI3K (thick arrow), but absolutely requires prior activation of the class IB PI3K. The steps leading from the initial PIP3 generation to class IA PI3K activation are not established (dashed lines). However, the Src family kinase inhibitor PP1 attenuates the second peak of PIP3, implicating this kinase family in the pathway. PIP3 and other PI3K products promote NADPH oxidase function.
Condliffe and colleagues demonstrate that in primed human neutrophils, fMLP induces a biphasic increase in PIP3 levels in which only the first peak is uniquely dependent on p110δ. The second peak is dependent on the first peak but also on the class IA PI3K isoform p110δ and, to a lesser extent, the class IA isoforms p110β and p110α (see figure). This suggests that neutrophil priming sets the stage for a sequential activation of distinct PI3K subtypes, a phenomenon previously observed in other leukocyte signaling systems.2 Although the mechanism has yet to be fully defined in this context, Src family kinases appear to be involved (see figure). The timing of ROS production correlates closely with the second peak of PIP3, and class IA-selective inhibitors are as potent as a class IB-selective inhibitor at blocking ROS. In primed mouse neutrophils, a similar biphasic PIP3 response was observed. However, in this case only the class IB inhibitor had major effects on lipid and ROS generation. In addition, PIP3 and ROS production were impaired in mouse neutrophils lacking p110γ but not in those with kinase-dead p110δ, the predominant class IA isoform in these cells. The conclusion that p110δ is dispensable for fMLP responses in murine neutrophils appears to conflict with work from another group,5 but may reflect different measurement time courses.
Pharmaceutical companies worldwide are actively developing isoform-selective PI3K inhibitors for various disease states including inflammatory conditions.3 The work of Condliffe and colleagues raises the possibility that class IA-selective inhibitors could be useful for minimizing ROS-mediated tissue damage in chronic inflammation. This work also highlights a potential limitation of murine neutrophils as a model system for studying signaling pathways regulating respiratory burst. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal