Abstract
Several primary murine and human B lymphomas and cell lines were found to constitutively express high levels of the activated form of c-jun N-terminal kinase (JNK), a member of the mitogen-activated protein (MAP) kinase family. Proliferation of murine B lymphomas CH31, CH12.Lx, BKS-2, and WEHI-231 and the human B lymphomas BJAB, RAMOS, RAJI, OCI-Ly7, and OCI-Ly10 was strongly inhibited by SP600125, an anthrapyrazolone inhibitor of JNK, in a dose-dependent manner. The lymphoma cells underwent apoptosis and arrested at the G2/M phase of cell cycle. Furthermore, JNK-specific small interfering RNA (siRNA) inhibited the growth of both murine and human B lymphomas. Thus in the B-lymphoma model, JNK appears to have a unique prosurvival role. Survival signals provided by CD40 and interleukin-10 (IL-10) together reversed the growth inhibition induced by the JNK inhibitor. c-Myc protein levels were reduced in the presence of both SP600125 and JNK-specific siRNA, and CD40 ligation restored c-Myc levels. Moreover, Bcl-xL rescued WEHI-231 cells from apoptosis induced by the JNK inhibitor. The JNK inhibitor also reduced levels of early growth response gene-1 (Egr-1) protein, and overexpressing Egr-1 partially rescued lymphoma cells from apoptosis. Thus, JNK may act via c-Myc and Egr-1, which were shown to be important for B-lymphoma survival and growth. (Blood. 2005;106:1382-1391)
Introduction
Jun N-terminal kinase (JNK; also known as stress-activated protein kinase, SAPK) is one of the 3 major members of the mitogen-activated protein kinase (MAPK) superfamily; the others are extracellular signal-regulated kinase (ERK) and the p38 MAP kinase. JNK is activated in response to certain growth factors or stresses such as ultraviolet (UV) radiation. Stress-induced JNK activation often leads to cell death through activation of the mitochondrial apoptotic pathway in many cell types including neuronal cells, prostate cancer cells, and fibroblasts.1-4 On the contrary, it has been shown recently that JNK can promote survival of BCR/ABL-transformed leukemic cells.5 Triggering the JNK pathway in vitro with a BCR-ABL tyrosine kinase led to a dramatic increase in B-cell transformation. Moreover, it was shown that JNK is required for interleukin-3 (IL-3)-mediated cell survival through its ability to phosphorylate and inactive the proapoptotic Bcl-2 family protein BAD.6 JNK protein kinases are coded for by 3 genes, Jnk1, Jnk2, and Jnk3. Jnk1 and Jnk2 are the more widely expressed isoforms of JNK. Jnk3 is limited in expression, restricted primarily to the brain, heart, and testis. JNK is activated by upstream MAPK kinases, MKK7 and MKK4.7-9 Activated JNK phosphorylates and activates its major substrate c-jun as well as several other transcription factors and proteins required for cell survival, proliferation, transformation, and cell death.10
The dual role of JNK in both apoptotic and survival signaling pathways indicates that the functional role of JNK is complex. The biologic outcome of JNK activation depends upon the cellular context, time course of activation, and the balance between the ability of JNK to signal both apoptosis and cell survival. The complexity of the cellular response to JNK activation can be illustrated by the diverse actions of a proinflammatory cytokine tumor necrosis factor alpha (TNF-α). Sustained activation of JNK correlates with TNF-induced apoptosis of rat mesangial cells.11 On the other hand, JNK1 and JNK2 double knock-out fibroblasts are more sensitive to TNF-induced apoptosis compared with wild-type fibroblasts, suggesting a prosurvival role for JNK signaling in these cells.12 Recent findings that MKK7 (an upstream activator of JNK) knock-out hepatocytes fail to proliferate and that mouse embryo fibroblasts that lack MKK7 undergo cellular senescence and G2/M growth arrest further support a role for JNK in cell-cycle progression.13
The role of JNK during primary B-lymphocyte growth responses still awaits complete illumination. Signaling through CD72, CD40, or B-cell receptor (BCR) ligation induces activation of MAP kinases, such as JNK, in primary splenic B cells.14-16 However, no defect in BCR- or CD72-induced proliferation is observed in B cells from JNK1-/- or JNK2-/- mice.14 This is probably due to a redundancy of function between the 2 isoforms, as JNK1 and JNK2 double knock outs exhibit embryonic lethality.17 In T cells, JNK2 is required for the differentiation of CD4+ T cells to T helper 1 (Th1) cells, and impaired interferon gamma (IFN-γ) production is observed in T cells from JNK2-/- mice.18 Using a dominant-negative mutant of TRAF2 (TNF receptor associated factor-2), it was shown that TRAF2 provides antiapoptotic signals by activating JNK following cross-linking of TNF receptor superfamily members in lymphocytes.19
Extensive work by several groups has established that MAP kinase pathways play critical roles in the pathogenesis of various hematologic malignancies, providing new molecular targets for future therapeutic approaches.20-22 Thus, inhibition of JNK activation with the pharmacologic JNK inhibitor SP600125 induces growth arrest in myeloma cell lines.23 Certain follicular lymphomas express constitutively the active form of p38 MAPK, and its inhibition with SB203580, the pharmacologic inhibitor, induces growth arrest and apoptosis.24 There is also evidence implicating abnormal expression of c-Jun, which is a downstream effector of the JNK pathway, in the proliferation of malignant Hodgkin lymphoma cells.25 Gene expression profiling of diffuse large B-cell lymphoma (DLBCL) revealed enhanced expression of JNK mRNA in at least 60% of the samples.26 In this context, it will be interesting to study the role of JNK MAPK in the growth regulation of B-cell lymphomas.
Here we demonstrate that JNK is constitutively activated in several B-lymphoma cell lines and primary lymphoma samples but not resting B cells. Using SP600125, as well as siRNA to knock down JNK activity, herein we show that JNK activation is required for primary B-cell proliferation in response to BCR cross-linking, and that basal JNK activity is required for the growth of a number of B lymphomas, including follicular, Burkitt, and DLBCLs.
Materials and methods
Reagents
SP600125 (Anthra[1,9-cd]pyrazol-6(2H)-one), an anthrapyrazolone inhibitor of JNK and a gift from Dr B. Bennett (Celgene, San Diego, CA), was dissolved in dimethyl sulfoxide (DMSO) and was then diluted to 2 mM in culture media as needed. Inhibitors of the ERK MAPK (PD98059 and U0126) and p38 MAPK (SB203580) were obtained from Calbiochem (San Diego, CA). Phospho-specific antibodies against JNKs (Thr183/Tyr185), ERKs (Thr202/Tyr204), and c-jun (Ser63) were obtained from Cell Signaling Technologies (Beverly, MA). Antibodies to JNK1, ERK, early growth response gene-1 (Egr-1, C-19), and c-Myc were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Monoclonal anti-β-actin antibody was obtained from Sigma (St Louis, MO). The 1C10 (anti-CD40) hybridoma was a gift from Dr M. Howard (Corixa, Redwood City, CA). Recombinant human IL-10 and murine IL-4, IL-5, IL-6, and TNF-α were obtained from R&D systems (Minneapolis, MN); and recombinant human IL-2, from the National Cancer Institute (Frederick, MD).
Cells and mice
The panel of B-cell lymphomas included cells of murine and human origin. Of murine origin were BKS-2, CH12.LX, CH31, WEHI-231, and the WEHI-231 Bcl-xL, and those of human origin included BJAB, Ramos, RAJI, and the human DLBCL cell lines OCI-Ly7 and OCI-Ly10. Cells from human lymphomas were obtained anonymously from discarded samples submitted for flow cytometry through an institutional review board (IRB)-approved protocol. The immature B-lymphoma cell line BKS-2 was isolated and maintained in vivo as splenic tumor in our laboratory.27 Female CBA/N (X-linked immunodeficient [Xid]) mice were obtained from Jackson Lab (Bar Harbor, ME). Mice were kept in microisolator cages in our American Association for Laboratory Animal Accreditation and Certification-approved rodent facility. BKS-2 B-lymphoma cells obtained from the spleens of CBA/N mice were depleted of T cells with a cocktail of anti-T-cell antibodies and complement as described.28 Normal splenic B cells were prepared according to procedures described previously.14 B cells were isolated from human peripheral blood mononuclear cells (PBMCs) by previously described procedures.29
Proliferation assay
BKS-2, BJAB, and Ramos cells were cultured in IF-12 medium (1:1 mixture of Iscove modified Dulbecco medium [IMDM] and Ham F12 (GIBCO, Grand Island, NY) + 10% fetal calf serum [FCS; Atlanta Biologicals, Norcross, GA]). DLBCL cell lines were cultured in IMDM + 20% normal human plasma. All other cell lines were cultured in RPMI supplemented with 10% FCS. To measure proliferation, 2 × 104 cells were cultured in 200 μL medium supplemented with 10% FCS. Cultures were treated with varying doses of SP600125, PD68059, or SB203580, or an equivalent concentration of DMSO. After 44 hours, the cultures were pulsed for 4 hours with 1 μCi (0.037 MBq) [3H] thymidine (DuPont/NEN, Boston, MA), and the cells were harvested onto filter mats using a cell harvester (Packard, Meriden, CT). The levels of radionucleotide incorporation were measured with a Matrix 96 β-radiation counter (Packard, Downers Grove, IL). Human B-cell cultures were pulsed at 66 hours and harvested 6 hours later. Results were presented as arithmetic mean of triplicate cultures plus or minus SE, and statistical significance of different treatments was evaluated by the Student t test. Percentage of control response was defined as counts per minute (cpm) in the treated group/cpm in the untreated group × 100.
Western blotting and flow cytometry
BKS-2 B-lymphoma cells, harvested from CBA/N mice and depleted of T cells, were treated with SP600125 at concentrations from 5 to 15 μM in 1-mL cultures of 1 × 106 cells in 6-well plates (Corning/Costar, Cambridge, MA). Cell lysates were prepared in 1 × sodium dodecyl sulfate (SDS) sample buffer or 1% Triton-X 100 as described earlier14,30 and were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis. The Western blots were analyzed by probing the membrane using various primary antibodies followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnologies). The blots were developed with Pico Chemiluminescence substrate (Pierce Biotechnology, Rockford, IL) and exposed to Kodak X-O mat film, which was scanned with a flat-bed scanner (UMAX Technologies, Hsinchu, Taiwan). Alternatively, the blots were scanned by a Kodak Image Station 2000RT (Eastman Kodak, New Haven, CT). For reprobing, membranes were stripped using a solution containing 62.5 mM Tris (tris(hydroxymethyl)aminomethane)-HCl, 2% SDS, and 100 mM β-mercaptoethanol at 65°C for 20 minutes. The relative integrated optical density (OD) of the protein bands was estimated using Scion Image software (Scion). Band intensities were normalized by dividing the intensity of phosphorylated protein by that of total protein in case of MAPK or by dividing protein of interest to β-actin. For flow cytometry, 1 × 106 cells were stained with B220-fluorescein isothiocyanate (FITC), annexin V-FITC, or biotinylated anti-intercellular adhesion molecule-1 (ICAM-1) + allophycocyanin (APC)-avidin (BD PharMingen, San Diego, CA).
Apoptosis and cell-cycle analysis
The cell-cycle status and apoptosis were analyzed using 2 methods: propidium iodide (PI) and Hoechst 33342 staining. Cultured B-lymphoma cells (1 × 106/2mL) were stained with 1 μg B220-FITC and then fixed in ethanol for at least 1 hour at 4°C, after which cells were incubated in a mixture of 1 μg/mL PI (Sigma) and 25 μg/mL RNase A (Sigma) at 37°C for at least 30 seconds. Hoechst 33342 staining was performed at 37°C (Molecular Probes, Eugene, OR) as described earlier.31 The level of PI or Hoechst 33342 fluorescence was measured with a MoFlo flow cytometer (DakoCytomation, Fort Collins, CO). Cells in the sub-G1 region were considered apoptotic.
In vitro kinase assay
To measure JNK activity, 200 μL of cell lysates were incubated with 20 μL of c-jun fusion protein beads overnight, and then kinase assay was performed per the manufacturer's instructions (Cell Signaling Technology). The reaction was carried out for 30 seconds at 30°C and was stopped by boiling the samples in 4 × SDS sample buffer. Levels of phospho-c-jun were measured by Western blot as described in “Western blotting and flow cytometry.”
Transfection of siRNA and plasmids into B-lymphoma cells
For transfection of siRNA, cells were washed and resuspended in Opti-MEM (low-serum medium; GIBCO, Grand Island, NY). Cells were electroporated with the green fluorescent protein (GFP) plasmid (pEGFP-N1) and the siRNA (control or JNK specific) at 250 mV, 960 μF, and 200 Ω with a Gene Pulser electroporator (BioRad, Hercules, CA). Three days after transfection, GFP+ cells were sorted by a fluorescence-activated cell sorter (FACS) MoFlo flow cytometer (DakoCytomation, Fort Collins, CO) and plated. Cell proliferation was measured at 24 hours and 48 hours after sorting, while some cells were lysed for Western blot. The target sequence for JNK-specific siRNA is 5′-AAAAAGAATGTCCTACCTTCT-3′, which is specific for both mice and humans.32 Both JNK-specific and control siRNA were obtained from Qiagen (Valencia, CA). For Egr-1 overexpression studies, BKS-2 B-lymphoma cells were transfected with GFP alone (pEGFP-N1) or cotransfected with GFP and Egr-1 expression vector (pBX-Egr) by electroporation as described earlier in this section.33 Two days later, cells were treated with 5 μM SP600125. Forty-eight hours after treatment, cells were analyzed for cell cycle and apoptosis.
In vivo studies
Female CBA/N mice were administered intravenously with 10 × 106 BKS-2 B-lymphoma cells on day 0. From day 1, mice were injected intraperitoneally either with 40 mg/kg body weight SP600125 (JNK inhibitor) or the vehicle (DMSO) alternate days for 10 days. On day 11, mice were killed and the number of nucleated cells was determined.
Results
Inhibition of JNK causes a dose-dependent reduction in primary splenic B-cell proliferation
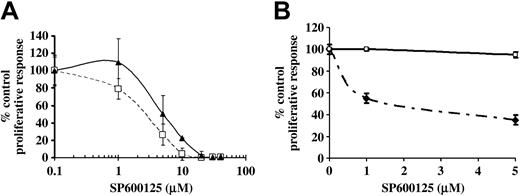
To test if JNK was necessary for proliferation of primary B cells, splenic B cells were stimulated with antibodies to BCR in the presence of varying concentrations of SP600125, the pharmacologic JNK inhibitor.34 A dose-dependent reduction in the proliferation of primary splenic B cells in response to BCR cross-linking was observed (Figure 1A). Supplementation with IL-4 did not reverse the SP600125-mediated inhibition of BCR-induced B-cell proliferation, although a small recovery was seen at low doses of the inhibitor (Figure 1A). We also observed a reduction in BCR-induced proliferative response of B cells from human peripheral blood upon JNK inhibition (Figure 1B). These data suggest that JNK is downstream of BCR-induced signaling pathways that lead to B-cell clonal expansion.
B-lymphoma cells constitutively express the activated form of JNK and its major substrate c-jun, and inhibition of basal JNK activity causes a dose-dependent reduction in their proliferation
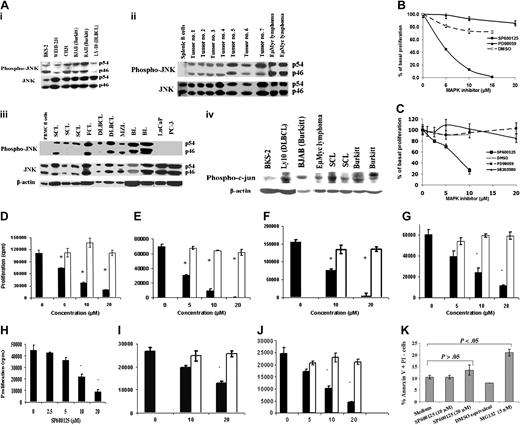
Next, we determined if JNK is constitutively activated in B-lymphoma cells that proliferate in the absence of BCR or other mitogenic signals. JNK (Figure 2Ai-iii) and its major substrate c-jun (Figure 2Aiv) were found to be constitutively phosphorylated in a variety of B-lymphoma cell lines and primary tumors of both murine and human origin (a total of 18 samples), whereas resting murine or human B cells contained little or no phosphorylated JNK (pJNK; Figure 2A). The prostate cancer lines LNCaP and PC-3 did not express the activated form of JNK, despite containing large amounts of the unphosphorylated form of JNK (Figure 2A third panel). This basal JNK activity was necessary for proliferation of B-lymphoma cells since treatment with varying concentrations of SP600125 induced a dose-dependent reduction in B-lymphoma proliferation, while the vehicle DMSO had minimal effects (Figure 2B-J). The BKS-2 B-lymphoma cells (follicular type) exhibited a 90% decrease in basal proliferation when treated with 10 μM SP600125. This appeared to be specific to the JNK inhibitor since no effect was observed when treated with the ERK inhibitor PD98059 or UO126 (data not shown) in this concentration range (Figure 2B). Growth of the human Burkitt lymphoma cell line BJAB was also reduced, showing a 70% decrease in basal proliferation compared with control when treated with SP600125, but showed no change in proliferation when treated with DMSO, PD98059, or SB203580, a p38 MAPK inhibitor (Figure 2C). Figure 2D-J shows the effect of SP600125 on additional murine B lymphomas (CH12-LX and CH31 [B-1-cell lymphomas] and WEHI-231 [an immature lymphoma]), human B lymphomas (Ramos and RAJI, 2 lines of Burkitt origin), and 2 in vitro-adapted DLBCL lines (OCI-Ly7 and OCI-Ly10). Similar results were also obtained with A20 (a mature B-cell lymphoma) and several Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines (data not shown), demonstrating that JNK was essential for proliferation of a variety of B-lymphoma cells. On the other hand, prostate cancer cell line PC-3 was relatively resistant to JNK inhibitor (Figure 2K) and did not express constitutively activated JNK (Figure 2A third panel). However, apoptosis was induced in PC-3 cells by a proteasome inhibitor (MG132) that prevents NF-κB activation.
Inhibition of JNK activity using SP600125 causes a reduction in BCR-induced proliferation of primary splenic B cells. (A) Splenic B cells from DBA/2 mice were treated with 50 μg/mL anti-IgM alone (□ and - - -) or 10 μg/mL anti-IgM + IL-4 20 U/mL (▴ and —) in the presence or absence of indicated doses of SP600125 for 2 days. Results were expressed as percentage of control response (mean ± SD of triplicate cultures) when compared with cells that were not treated with SP600125 (the actual counts are 81 032 ± 12 381 for anti-IgM 50 μg/mL, 16 489 ± 3815 for anti-IgM plus IL-4). (B) Human peripheral blood B cells were treated with 10 μg/mL anti-IgM in the absence (□) or presence (♦) of indicated doses of SP600125 for 3 days. The actual counts were 4942 ± 61 for anti-IgM (10 μg/mL) + IL-2 (100 U/mL) and 4717 ± 208 for DMSO equivalent. Proliferation was measured as described in “Materials and methods.” Results are presented as mean ± SE of triplicate cultures. Results are representative of 4 experiments in panel A and of 2 experiments in panel B.
Inhibition of JNK activity using SP600125 causes a reduction in BCR-induced proliferation of primary splenic B cells. (A) Splenic B cells from DBA/2 mice were treated with 50 μg/mL anti-IgM alone (□ and - - -) or 10 μg/mL anti-IgM + IL-4 20 U/mL (▴ and —) in the presence or absence of indicated doses of SP600125 for 2 days. Results were expressed as percentage of control response (mean ± SD of triplicate cultures) when compared with cells that were not treated with SP600125 (the actual counts are 81 032 ± 12 381 for anti-IgM 50 μg/mL, 16 489 ± 3815 for anti-IgM plus IL-4). (B) Human peripheral blood B cells were treated with 10 μg/mL anti-IgM in the absence (□) or presence (♦) of indicated doses of SP600125 for 3 days. The actual counts were 4942 ± 61 for anti-IgM (10 μg/mL) + IL-2 (100 U/mL) and 4717 ± 208 for DMSO equivalent. Proliferation was measured as described in “Materials and methods.” Results are presented as mean ± SE of triplicate cultures. Results are representative of 4 experiments in panel A and of 2 experiments in panel B.
JNK inhibition induces growth arrest and apoptosis in B-lymphoma cells
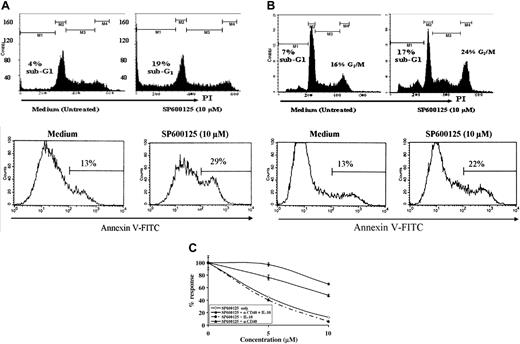
We investigated whether the decrease in proliferation was due to growth arrest, apoptosis, or both. Cell-cycle analysis performed by staining DNA with PI or Hoechst yielded similar results, both demonstrating an increase in sub G1 population, an indicator of apoptotic cells (Figure 3A top panel). The apoptotic population in BKS-2 increased from 4% ± 1% to 19% ± 2% when treated with SP600125. We also measured apoptosis by annexin V staining. Annexin V-positive cells increased from 13% ± 1% to 29% ± 2% when BKS-2 B lymphomas were treated with 10 μM SP600125 (Figure 3A bottom panel). A similar increase in apoptotic cells was observed in WEHI-231, another murine B lymphoma (PI staining, Figure 3B top panel; or annexin V staining, Figure 3B bottom panel). Of interest, WEHI-231 and CH31 B-lymphoma cells not only exhibit enhanced apoptosis, but also undergo G2/M arrest when stained with PI (Figure 3B; data not shown). Similar G2/M arrest was also seen in BKS-2 lymphoma at higher concentrations of SP600125. The majority of B lymphomas of human origin predominantly underwent G2/M growth arrest with fewer apoptotic cells (Table 1).
Cell-cycle analysis of human Burkitt B-lymphoma cells treated with SP600125 inhibitor for 48 hours
Cell line . | Sub-G1 (apoptotic) . | G0/G1 . | S . | G2/M . |
|---|---|---|---|---|
| BJAB (medium) | 1 ± 0 | 42 ± 0 | 25 ± 0 | 32 ± 0 |
| BJAB (SP600125, 20 μM) | 3 ± 0 | 18 ± 1 | 15 ± 1 | 64 ± 2 |
| RAJI (medium) | 6 ± 1 | 39 ± 0 | 38 ± 1 | 16 ± 0 |
| RAJI (SP600125, 20 μM) | 13 ± 1 | 22 ± 1 | 30 ± 2 | 33 ± 2 |
Cell line . | Sub-G1 (apoptotic) . | G0/G1 . | S . | G2/M . |
|---|---|---|---|---|
| BJAB (medium) | 1 ± 0 | 42 ± 0 | 25 ± 0 | 32 ± 0 |
| BJAB (SP600125, 20 μM) | 3 ± 0 | 18 ± 1 | 15 ± 1 | 64 ± 2 |
| RAJI (medium) | 6 ± 1 | 39 ± 0 | 38 ± 1 | 16 ± 0 |
| RAJI (SP600125, 20 μM) | 13 ± 1 | 22 ± 1 | 30 ± 2 | 33 ± 2 |
Values presented are percentage of cells in each phase of cell cycle. Phase of cell cycle was determined by flow cytometric analysis of propidium iodide-stained cells. Results are presented as means ± SE of triplicate cultures. Experiments were performed 2 to 3 times with similar outcome.
Constitutive expression of activated JNK and phospho-c-jun in B lymphomas and the effect of MAPK inhibitors on the growth of murine and human B-lymphoma cells. (A) Murine and human B-lymphoma cell lines (i), primary B lymphoma isolated from mouse (ii), and primary B-lymphoma samples from human patients (iii) expressed phosphorylated form of JNK constitutively with little expression by normal splenic B cells, normal peripheral blood human B cells, and prostate cancer cells (LNCaP and PC-3). A variety of B-lymphoma cell lines and tumors from both murine and human origin express phosphorylated form of c-jun constitutively (iv). Mouse primary tumors are spontaneous B lymphomas from aged mice (tumor nos. 1 to 7) and B lymphomas isolated from Eμ-Myc transgenic mice. Human primary B lymphomas include small-cell lymphoma (SCL), large-cell lymphoma (LCL), follicular cell lymphoma (FCL), Burkitt lymphoma (BL), and marginal zone lymphoma (MZL), which are characterized by flow cytometry. (B) BKS-2 B-lymphoma cells were cultured for 48 hours with vehicle (DMSO) alone or with indicated concentrations of SP600125 or PD98059. Results were expressed as percentage of basal proliferation (mean ± SE of triplicate cultures) when compared with cells that were not treated with any inhibitor. The actual counts are 61 449 ± 2636 for the untreated cells. (C) BJAB B-lymphoma cells were cultured for 48 hours with vehicle (DMSO) alone, or the indicated concentrations of SP600125, PD98059, or SB203580. The actual counts are 119 333 ± 7118 for the medium. Panels D, E, F, G, and H represent the effect of SP600125 on basal proliferation of CH12.LX, WEHI-231, CH31, Ramos, and RAJI B-lymphoma cells, respectively; panels I and J, of OCI-Ly7 and OCI-Ly10 DLBCL cells, respectively. For D-G and I-J, ▪ indicates SP600125; □, DMSO equivalents. (K) Annexin V staining of prostate cancer cell line PC-3 in the presence or absence of varying concentrations of SP600125 for 24 hours. MG132 is used as a positive control, which is a proteasome inhibitor. Results are presented as means ± SE of triplicate cultures. *P < .05 when comparing response with SP600125 to solvent or medium-only treatment. Results are representative of 3 experiments.
Constitutive expression of activated JNK and phospho-c-jun in B lymphomas and the effect of MAPK inhibitors on the growth of murine and human B-lymphoma cells. (A) Murine and human B-lymphoma cell lines (i), primary B lymphoma isolated from mouse (ii), and primary B-lymphoma samples from human patients (iii) expressed phosphorylated form of JNK constitutively with little expression by normal splenic B cells, normal peripheral blood human B cells, and prostate cancer cells (LNCaP and PC-3). A variety of B-lymphoma cell lines and tumors from both murine and human origin express phosphorylated form of c-jun constitutively (iv). Mouse primary tumors are spontaneous B lymphomas from aged mice (tumor nos. 1 to 7) and B lymphomas isolated from Eμ-Myc transgenic mice. Human primary B lymphomas include small-cell lymphoma (SCL), large-cell lymphoma (LCL), follicular cell lymphoma (FCL), Burkitt lymphoma (BL), and marginal zone lymphoma (MZL), which are characterized by flow cytometry. (B) BKS-2 B-lymphoma cells were cultured for 48 hours with vehicle (DMSO) alone or with indicated concentrations of SP600125 or PD98059. Results were expressed as percentage of basal proliferation (mean ± SE of triplicate cultures) when compared with cells that were not treated with any inhibitor. The actual counts are 61 449 ± 2636 for the untreated cells. (C) BJAB B-lymphoma cells were cultured for 48 hours with vehicle (DMSO) alone, or the indicated concentrations of SP600125, PD98059, or SB203580. The actual counts are 119 333 ± 7118 for the medium. Panels D, E, F, G, and H represent the effect of SP600125 on basal proliferation of CH12.LX, WEHI-231, CH31, Ramos, and RAJI B-lymphoma cells, respectively; panels I and J, of OCI-Ly7 and OCI-Ly10 DLBCL cells, respectively. For D-G and I-J, ▪ indicates SP600125; □, DMSO equivalents. (K) Annexin V staining of prostate cancer cell line PC-3 in the presence or absence of varying concentrations of SP600125 for 24 hours. MG132 is used as a positive control, which is a proteasome inhibitor. Results are presented as means ± SE of triplicate cultures. *P < .05 when comparing response with SP600125 to solvent or medium-only treatment. Results are representative of 3 experiments.
Growth inhibitory effects of JNK inhibition can be overcome by addition of α-CD40 and IL-10
To test whether the effects of JNK inhibition by SP600125 could be reversed, we treated lymphoma cells with a combination of anti-CD40 and IL-10. It is known that ligation of CD40 on the surface of primary B cells and some B-lymphoma cells can enhance their proliferation. IL-10, produced by many B-cell lymphomas, has been shown to promote survival of B lymphomas.35-37 A combination of anti-CD40 and IL-10 was able to overcome growth inhibition caused by SP600125 (Figure 3C). Treatment with IL-10 alone did not reverse SP600125-induced apoptosis, whereas treatment with α-CD40 alone was only partially effective. This combination is unique since TNF-α, IL-1, IL-2, IL-4, IL-5, and IL-6 failed to rescue BKS-2 cells from SP600125-induced growth inhibition, nor did they enhance the partial effect of anti-CD40 (data not shown).
Inhibition of JNK activity induces growth arrest and apoptosis in B-lymphoma cells and can be overcome by the addition of anti-CD40 and IL-10. (A) BKS-2 murine B-lymphoma cells were cultured in 6-well plates at 1 × 106 cells/well in the presence of 0 to 10 μM doses of SP600125. Two days later, cell-cycle analysis was performed on these cultured cells by PI staining as described in “Materials and methods” (top). (Bottom) Histogram of annexin V-positive cells gated on PI-negative population in the presence or absence of 10 μM SP600125. (B, top) Histogram representation of PI staining of WEHI-231 B lymphoma in the presence or absence of the JNK inhibitor. (Bottom) Histogram of annexin V-positive cells gated on PI-negative population in the presence or absence of 10 μM SP600125. In top panels, M1, M2, M3, and M4 indicate cells in sub-G1, G1, S, and G2/M phases of cell cycle. In bottom panels, percentages indicate annexin V-positive cells. (C) BKS-2 B-lymphoma cells were incubated with different doses of SP600125 in the presence or absence of α-CD40 (1:1000 dilution of ascites) and IL-10 (50 U/mL), or vehicle (DMSO) alone for 2 days, and proliferation was measured as described in “Materials and methods.” Results were expressed as percentage of control response (mean ± SE of triplicate cultures) when compared with cells that were not treated with any inhibitor. Experiments were done 2 to 3 times with similar results.
Inhibition of JNK activity induces growth arrest and apoptosis in B-lymphoma cells and can be overcome by the addition of anti-CD40 and IL-10. (A) BKS-2 murine B-lymphoma cells were cultured in 6-well plates at 1 × 106 cells/well in the presence of 0 to 10 μM doses of SP600125. Two days later, cell-cycle analysis was performed on these cultured cells by PI staining as described in “Materials and methods” (top). (Bottom) Histogram of annexin V-positive cells gated on PI-negative population in the presence or absence of 10 μM SP600125. (B, top) Histogram representation of PI staining of WEHI-231 B lymphoma in the presence or absence of the JNK inhibitor. (Bottom) Histogram of annexin V-positive cells gated on PI-negative population in the presence or absence of 10 μM SP600125. In top panels, M1, M2, M3, and M4 indicate cells in sub-G1, G1, S, and G2/M phases of cell cycle. In bottom panels, percentages indicate annexin V-positive cells. (C) BKS-2 B-lymphoma cells were incubated with different doses of SP600125 in the presence or absence of α-CD40 (1:1000 dilution of ascites) and IL-10 (50 U/mL), or vehicle (DMSO) alone for 2 days, and proliferation was measured as described in “Materials and methods.” Results were expressed as percentage of control response (mean ± SE of triplicate cultures) when compared with cells that were not treated with any inhibitor. Experiments were done 2 to 3 times with similar results.
SP600125 inhibits phosphorylation of c-jun but not ERK or p38 MAPK
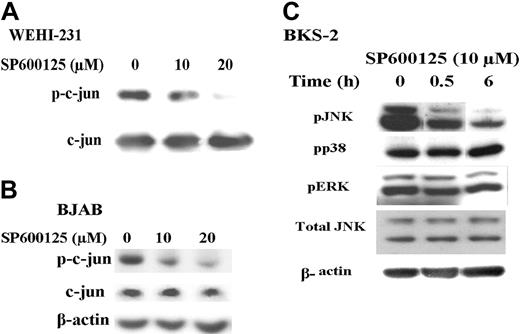
To verify the specificity of the JNK inhibitor SP600125, we performed an in vitro kinase assay using c-jun fusion protein beads and cold adenosine triphosphate (ATP). Cells were treated for 6 hours with SP600125, and total cell lysates were subjected to in vitro kinase assay as described in “Materials and methods.” There is a dose-dependent reduction in the phosphorylation of c-jun in the presence of SP600125 (Figure 4A). Consistent with the presence of constitutively activated pJNK in several B lymphomas shown in Figure 2, phosphorylated c-jun was detected in untreated cells (Figure 4B) and treatment with SP600125 for 6 hours reduced the levels of phospho-c-jun. Similar results were obtained with the murine B-lymphoma cell lines. Treatment with SP600125 did not reduce the levels of pERK or phospho-p38 as shown in Figure 4C. However, SP600125 partially inhibits phosphorylation of JNK (Figure 4C), which is consistent with published results.38-40
Knocking down JNK by RNA interference causes a decrease in proliferation and an increase in apoptosis of B-lymphoma cells but not prostate cancer cells
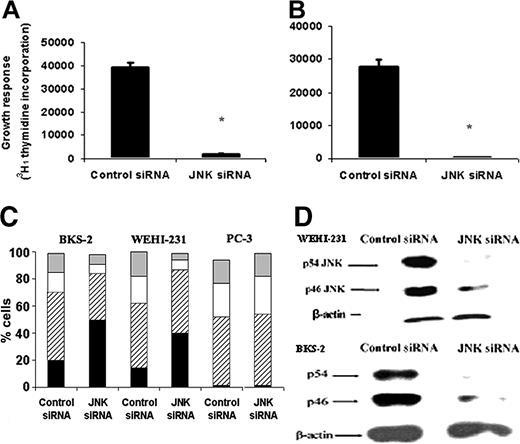
As an alternate approach to test the significance of JNK for lymphoma growth, we targeted JNK using siRNA that can recognize a common sequence in both JNK1 and JNK2 from both mice and humans.32 As shown in Figure 5A, there was a 90% decrease in basal proliferation in BKS-2 B-lymphoma cells transfected with JNK-specific siRNA compared with control siRNA-transfected cells. We observed a similar growth inhibition in the WEHI-231 B-lymphoma cells (Figure 5B) and BJAB (data not shown). Figure 5C showed that there was more apoptosis and a significant decrease in S phase in BKS-2 and WEHI-231 cells transfected with JNK-specific siRNA compared with control. Western analysis showed that siRNA treatment reduced JNK protein levels in both BKS-2 and WEHI-231 lymphoma cells (Figure 5D). Inhibiting constitutive JNK activity by 2 different approaches, namely SP600125 and siRNA, suggested a prosurvival role for JNK in B-lymphoma cells. On the other hand, treating prostate cancer cell line PC-3, which lacks constitutive JNK expression (Figure 2A third panel), with JNK-specific siRNA did not induce apoptosis as measured by PI analysis (Figure 5C).
SP600125 inhibits c-jun phosphorylation but not phosphorylation of ERK or p38 MAPK. (A) WEHI-231 B-lymphoma cells were incubated with medium or treated with 10 μM SP600125 for 6 hours. In vitro kinase assays were performed on the cell lysates and subsequently analyzed by immunoblotting with an antibody to phospho-c-jun. (B) BJAB B-lymphoma cells were incubated with medium or treated with 10 μM SP600125 for 6 hours. Cell lysates were analyzed by immunoblotting with an antibody to phospho-c-jun. (C) BKS-2 B-lymphoma cells were incubated with medium or treated with 10 μM SP600125 for 6 hours. Cell lysates were analyzed by immunoblotting with an antibody to phospho-JNK, phospho-ERK, or phospho-p38. All the blots that were probed for phosphokinases were then stripped and reprobed with antibodies to total kinase or actin to correct for changes in protein loading in different lanes. Experiments were performed 2 times with similar results.
SP600125 inhibits c-jun phosphorylation but not phosphorylation of ERK or p38 MAPK. (A) WEHI-231 B-lymphoma cells were incubated with medium or treated with 10 μM SP600125 for 6 hours. In vitro kinase assays were performed on the cell lysates and subsequently analyzed by immunoblotting with an antibody to phospho-c-jun. (B) BJAB B-lymphoma cells were incubated with medium or treated with 10 μM SP600125 for 6 hours. Cell lysates were analyzed by immunoblotting with an antibody to phospho-c-jun. (C) BKS-2 B-lymphoma cells were incubated with medium or treated with 10 μM SP600125 for 6 hours. Cell lysates were analyzed by immunoblotting with an antibody to phospho-JNK, phospho-ERK, or phospho-p38. All the blots that were probed for phosphokinases were then stripped and reprobed with antibodies to total kinase or actin to correct for changes in protein loading in different lanes. Experiments were performed 2 times with similar results.
Effects of JNK-specific siRNA on basal proliferation and survival of B-lymphoma cells. (A) BKS-2 or (B) WEHI-231 B-lymphoma cells were cotransfected with the GFP plasmid and siRNA (control or JNK specific). Three days later, GFP+ cells were sorted by FACS and proliferation assay was performed as described in “Materials and methods.” Results are presented as means ± SE of triplicate cultures. (C) Hoechst analysis of GFP+ BKS-2 and WEHI-231 B-lymphoma cells or prostate cancer cells PC-3 cotransfected with control or JNK-specific siRNA. ▦ indicates G2/M; □, S; ▨, G1; and ▪, sub-G1. (D) Western analysis of lysates from control or JNK-specific siRNA-treated WEHI-231 (top) and BKS-2 cells (bottom) by an antibody to JNK. The blots were then stripped and reprobed for β-actin as a loading control. Results are representative of 2 to 3 experiments. *P < .001 when response with JNK siRNA is compared with control siRNA.
Effects of JNK-specific siRNA on basal proliferation and survival of B-lymphoma cells. (A) BKS-2 or (B) WEHI-231 B-lymphoma cells were cotransfected with the GFP plasmid and siRNA (control or JNK specific). Three days later, GFP+ cells were sorted by FACS and proliferation assay was performed as described in “Materials and methods.” Results are presented as means ± SE of triplicate cultures. (C) Hoechst analysis of GFP+ BKS-2 and WEHI-231 B-lymphoma cells or prostate cancer cells PC-3 cotransfected with control or JNK-specific siRNA. ▦ indicates G2/M; □, S; ▨, G1; and ▪, sub-G1. (D) Western analysis of lysates from control or JNK-specific siRNA-treated WEHI-231 (top) and BKS-2 cells (bottom) by an antibody to JNK. The blots were then stripped and reprobed for β-actin as a loading control. Results are representative of 2 to 3 experiments. *P < .001 when response with JNK siRNA is compared with control siRNA.
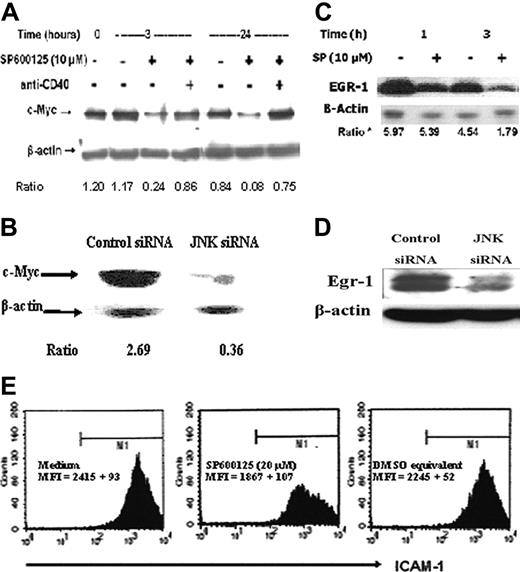
Cell-cycle regulator c-Myc and prosurvival transcription factor Egr-1 are downstream of JNK signaling in B-lymphoma cells. (A) WEHI-231 B-lymphoma cells were incubated with medium, 10 μM SP600125, and anti-CD40, either alone or in combination for the indicated time periods. Cell lysates were analyzed by immunoblotting with an antibody to c-Myc. The blots were then stripped and probed with anti-β-actin antibody to correct for changes in protein loading in different lanes. (B) Immunoblotting of lysates from control or JNK-specific siRNA-transfected WEHI-231 cells with antibodies to c-Myc and β-actin. (C) BKS-2 B-lymphoma cells were incubated with medium or 10 μM SP600125 for the indicated time periods. Cell lysates were analyzed by immunoblotting with an antibody to Egr-1 and then stripped and probed for β-actin. (D) WEHI-231 B-lymphoma cells were treated with control or JNK-specific siRNA for 24 hours. Cell lysates were analyzed by immunoblotting with an antibody to Egr-1 and then stripped and probed for β-actin. (E) Flow cytometry analysis of SP600125- or vehicle (DMSO)-treated BKS-2 cells with an antibody to ICAM-1. Flow cytometry was performed as described in “Materials and methods.” Numbers indicate the ratio of density of c-Myc to β-actin in panels A and B and of Egr-1 to β-actin in panel C. Experiments were done 2 to 3 times with similar results.
Cell-cycle regulator c-Myc and prosurvival transcription factor Egr-1 are downstream of JNK signaling in B-lymphoma cells. (A) WEHI-231 B-lymphoma cells were incubated with medium, 10 μM SP600125, and anti-CD40, either alone or in combination for the indicated time periods. Cell lysates were analyzed by immunoblotting with an antibody to c-Myc. The blots were then stripped and probed with anti-β-actin antibody to correct for changes in protein loading in different lanes. (B) Immunoblotting of lysates from control or JNK-specific siRNA-transfected WEHI-231 cells with antibodies to c-Myc and β-actin. (C) BKS-2 B-lymphoma cells were incubated with medium or 10 μM SP600125 for the indicated time periods. Cell lysates were analyzed by immunoblotting with an antibody to Egr-1 and then stripped and probed for β-actin. (D) WEHI-231 B-lymphoma cells were treated with control or JNK-specific siRNA for 24 hours. Cell lysates were analyzed by immunoblotting with an antibody to Egr-1 and then stripped and probed for β-actin. (E) Flow cytometry analysis of SP600125- or vehicle (DMSO)-treated BKS-2 cells with an antibody to ICAM-1. Flow cytometry was performed as described in “Materials and methods.” Numbers indicate the ratio of density of c-Myc to β-actin in panels A and B and of Egr-1 to β-actin in panel C. Experiments were done 2 to 3 times with similar results.
Cell-cycle regulator c-Myc is down-regulated upon inhibition of JNK signaling in B-lymphoma cells
Several studies have shown that c-Myc is an important oncoprotein that is overexpressed in many human and murine B lymphomas. Dysregulated c-Myc expression was critical for proliferation of such lymphomas, and transgenic expression of c-Myc in B cells resulted in B-lymphoma development.41-47 In WEHI-231 and BKS-2 cells, BCR cross-linking led to down-regulation of c-Myc, resulting in growth inhibition that was overcome by c-Myc overexpression.28,48 So, we investigated whether levels of c-Myc were affected by the JNK inhibitor. Western analysis of lysates obtained from WEHI-231 B lymphoma treated with 10 μM SP600125 showed a reduction in c-Myc protein levels as early as 3 hours that persisted even at 24 hours compared with untreated cells (Figure 6A). When JNK was inhibited with JNK-specific siRNA, an 87% reduction in c-Myc protein levels was observed in WEHI-231 cells, confirming the specific effect of the JNK inhibitor (Figure 6B). Reduced c-Myc expression was also observed in BKS-2 and BJAB B-lymphoma cells when treated with SP600125 (data not shown). Moreover, c-Myc levels were restored by signaling through CD40 in the presence of the JNK inhibitor (Figure 6A) in accordance with the ability of CD40 to overcome the JNK inhibitor-induced growth inhibition (Figure 3C).
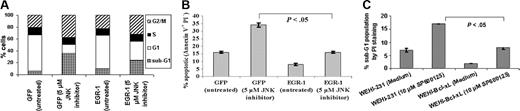
Egr-1 partially rescues BKS-2 B-lymphoma cells from SP600125-induced apoptosis, and Bcl-xL antagonizes apoptosis induced by SP600125 in WEHI-231 B-lymphoma cells. (A) Hoechst analysis of BKS-2 cells expressing control vector (GFP alone) or Egr-1 in the presence or absence of 5 μM SP600125 at 48 hours. (B) Same as in panel A except for analysis by annexin V and PI staining for apoptotic cells. (C) PI analysis of WEHI-231 or WEHI-231 stably transfected with Bcl-xL (WEHI-Bcl-xL) in the presence or absence of 10 μM SP600125. Results are presented as means ± SE of triplicate cultures. Similar results were obtained in 2 other experiments.
Egr-1 partially rescues BKS-2 B-lymphoma cells from SP600125-induced apoptosis, and Bcl-xL antagonizes apoptosis induced by SP600125 in WEHI-231 B-lymphoma cells. (A) Hoechst analysis of BKS-2 cells expressing control vector (GFP alone) or Egr-1 in the presence or absence of 5 μM SP600125 at 48 hours. (B) Same as in panel A except for analysis by annexin V and PI staining for apoptotic cells. (C) PI analysis of WEHI-231 or WEHI-231 stably transfected with Bcl-xL (WEHI-Bcl-xL) in the presence or absence of 10 μM SP600125. Results are presented as means ± SE of triplicate cultures. Similar results were obtained in 2 other experiments.
Prosurvival transcription factor Egr-1 is down-regulated upon JNK inhibition, and its overexpression partially protects against apoptosis induced by the JNK inhibitor
Previous studies from our laboratory have shown that Egr-1 was overexpressed in BKS-2 B lymphoma and that inhibition of Egr-1 using antisense oligonucleotides specific for Egr-1 induced apoptosis.49 Based on this finding, we hypothesized that Egr-1 might be downstream of JNK signaling. There was a reduction in the Egr-1 protein in cells treated with 10 μM SP600125 (Figure 6C) or with JNK siRNA (Figure 6D). Moreover, the Egr-1 target gene ICAM-1 levels were slightly reduced but in a highly reproducible manner in SP600125-treated cells (83% of control cells; Figure 6E).50,51 Egr-1 and GFP were ectopically expressed by cotransfection with 2 plasmids. Cell-cycle analysis of GFP-positive cells demonstrated that Egr-1 expression partially overcame the inhibitory effects of SP600125 (Figure 7A). Direct examination of apoptosis with annexin V further confirmed the protective effect of Egr-1 (Figure 7B). However, Egr-1 expression failed to reverse the G2/M arrest (Figure 7A).
Bcl-xL overcomes SP600125-induced apoptosis in WEHI-231 B-lymphoma cells
Previously, we showed that Egr-1 inhibition was accompanied by a decrease in Bcl-xL expression in BKS-2 cells.52 Moreover, Bcl-xL is overexpressed in some B lymphomas and can be induced by CD40 signaling.53-55 Anti-IgM-induced apoptosis of WEHI-231 cells is prevented by stable expression of Bcl-xL.56 Accordingly, we found that WEHI-231 cells that stably express Bcl-xL were resistant to apoptosis induced by the JNK inhibitor compared with WEHI-231 cells (Figure 7C), but they continued to exhibit G2/M arrest (data not shown).
In vivo role for JNK in B-lymphoma growth regulation
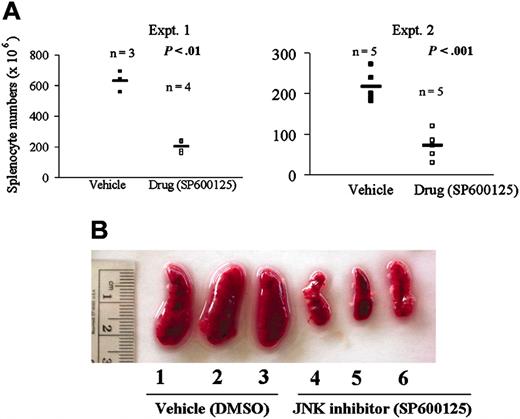
Having shown that JNK plays a prosurvival role in regulating B-lymphoma growth in vitro, we investigated the role of JNK in vivo in our mouse model of B-lymphoma BKS-2. There was a significant reduction in the tumor burden of drug (SP600125)-treated but not vehicle (DMSO)-treated mice as measured by the total splenocyte numbers (Figure 8A). Representative spleens from both vehicle- and drug-treated mice are shown in Figure 8B.
Inhibiting JNK activity with SP600125 leads to regression of B-lymphoma growth in vivo. (A) Splenocyte numbers of mice treated with vehicle (DMSO) or drug (SP600125). Mice were administered 10 × 106 BKS-2 B-lymphoma cells on day 0 and subsequently with intraperitoneal injection of 40 mg/kg body weight of the drug SP600125 or the vehicle (DMSO) alternate days for 10 days. Experiments (Expt.) were performed 2 times. (B) Photograph of vehicle- and drug-treated spleen of CBA/N mice on day 11. Horizontal bars represent means of all the numbers for all the mice in the group.
Inhibiting JNK activity with SP600125 leads to regression of B-lymphoma growth in vivo. (A) Splenocyte numbers of mice treated with vehicle (DMSO) or drug (SP600125). Mice were administered 10 × 106 BKS-2 B-lymphoma cells on day 0 and subsequently with intraperitoneal injection of 40 mg/kg body weight of the drug SP600125 or the vehicle (DMSO) alternate days for 10 days. Experiments (Expt.) were performed 2 times. (B) Photograph of vehicle- and drug-treated spleen of CBA/N mice on day 11. Horizontal bars represent means of all the numbers for all the mice in the group.
Discussion
We reported earlier that primary splenic B cells express activated forms of JNK when either the BCR or CD72 (a coreceptor on B cells) is ligated, stimuli that induce normal B-cell proliferation.14 Using a pharmacologic inhibitor, we herein show that JNK activity is essential for BCR-induced proliferation of both normal murine splenic B cells and peripheral blood human B cells. Furthermore, we found that many B-lymphoma cell lines as well as primary tumor samples of both human and murine origin constitutively express the activated form of JNK (pJNK) and its major substrate c-jun (phospho-c-jun). This basal JNK activity is required for B-lymphoma proliferation. Inhibition of JNK activation with SP600125 or inhibition of JNK expression by siRNA resulted in reduced basal proliferation of several murine B-lymphoma cell lines, human Burkitt B-lymphoma cells, as well as human DLBCL cell lines. Our findings for the first time demonstrate that JNK activity is critical for B-lymphoma proliferation. In this lymphoma cell system, the SP600125 inhibitor appeared to be highly specific to JNK since phosphorylation of ERK and p38 MAP kinases was not affected. Moreover, SP600125 has been shown to specifically inhibit JNK activity without altering the activity of cyclin A/cyclin-dependent kinase (cdk), Akt, signal transducer and activator of transcription 3 (STAT3), and p27, and several other kinases in in vitro systems.23,57,58 This is further supported by our observations that the prostate cancer cell line PC-3, which lacks constitutively activated JNK, is resistant to SP600125-induced growth inhibition. Western analysis with isoform-specific antibodies showed that both JNK1 and JNK2 are expressed in these lymphoma cells. The relative contribution of the 2 isoforms of JNK requires further investigation.
The growth inhibitory effect of SP600125 can be reversed by simultaneous stimulation through anti-CD40 and IL-10. Ligation of CD40 transmits signals that abrogate apoptosis induced by BCR ligation in immature B-lymphoma cell lines such as WEHI-231.59 Expression of Bcl-xL and A1 is enhanced by CD40 ligation, suggesting a role for antiapoptotic proteins in CD40-induced B-cell survival.60-62 Accordingly, we found that JNK inhibition did not induce apoptosis in Bcl-xL-expressing WEHI-231 cells. IL-10, a pleiotropic cytokine with anti-inflammatory properties, is a potent growth and survival factor for malignant B cells. The New Zealand black (NZB) strain of mice develop an age-related malignant expansion of B-1 cells with autocrine production of IL-10. Of interest, the IL-10 knock-out mice on NZB background do not develop malignancy, suggesting that IL-10 is a critical factor for the progression of this B-cell-malignant disease.63 Our data show that the growth inhibitory effects of JNK inhibition can be rescued by signaling through both CD40 and IL-10 receptor.
We also tested the significance of 2 other MAPK family members, including ERK and p38, in B-lymphoma growth regulation. Although these B lymphomas express activated forms of ERK constitutively, inhibition of its activity by 2 different inhibitors PD98059 and UO126 did not affect their basal proliferation. Moreover, the p38 inhibitor SB203580 did not inhibit the proliferation of 2 B-lymphoma cell lines tested. These data demonstrate that ERK and p38 MAPK are not crucial for basal proliferation of B-lymphoma cell lines BKS-2 and BJAB. However, p38 MAP kinase has recently been shown to be activated during progression of follicular lymphoma into DLCBL.24 Hence more B lymphomas have to be examined for the relative importance of different MAPK enzymes for lymphoma growth.
Decrease in proliferation can be due to increased growth arrest, apoptosis, or both. The fact that more cells shift from S phase toward sub-G1 phase of cell cycle in the presence of either SP600125 or the JNK-specific siRNA in BKS-2 B-lymphoma cells suggests that these cells undergo apoptosis. Of interest, all the lymphoma cells undergo G2/M growth arrest in addition to apoptosis. We also observed aneuploidy by PI analysis when WEHI-231 and CH31 B-lymphoma cells were treated with 20 μM or more of SP600125. In fact, similar observations have been made in multiple myeloma cells, erythroleukemic cells, and breast cancer cells, where SP600125 induces both G2/M arrest and apoptosis.23,57,58 This is consistent with an increase in JNK activity during G2/M phase of cell cycle observed in Jurkat and carcinoma cells.64,65 The apoptosis may be secondary to G2/M arrest since Bcl-xL expression overcomes apoptosis but not cell-cycle arrest.
Many B lymphomas overexpress c-Myc due to chromosomal translocations, and this overexpression is important for their proliferation and cell-cycle progression.41,42,46,48,66 JNK is known to up-regulate c-Myc expression in response to growth factors such as platelet-derived growth factor.67 Moreover, it has been shown that WEHI-231 B-lymphoma cells stably transfected with c-Myc are resistant to anti-IgM-induced growth arrest.48 We observed a significant reduction in c-Myc protein in both SP600125- and siRNA-treated WEHI-231 B lymphoma, suggesting that inhibition of JNK may cause growth arrest by inhibiting c-Myc expression. c-Myc translocation, which leads to its overexpression, is common in Burkitt B-lymphoma cells. However, in Burkitt lymphomas, often the c-Myc gene is translocated to the immunoglobulin heavy-chain locus, whose expression may still be regulated by the MAPK pathway. This may account for the growth inhibition of these cells by the JNK inhibitor.
c-Myc expression has been shown to be cell-cycle dependent. Elevated c-Myc levels were observed during G1 and G2/M phases of cell cycle with moderate levels at the S phase, and c-Myc was shown to cooperate with Ras to induce cdc2, a kinase required for G2/M progression.68 Moreover, it was shown that drug-induced G2/M arrest of Jurkat T cells is correlated with c-Myc down-regulation.69 It is possible that JNK regulates c-Myc during the G2/M phase of cell-cycle progression since MKK7 knock-out fibroblasts in which JNK activation is compromised undergo G2/M arrest and cellular senescence.13 Down-regulated c-Myc might account for the G2/M arrest observed in SP600125-treated cells. This is consistent with the protective effect of CD40, as CD40 signaling restored c-Myc levels (Figure 6).56 Moreover, our preliminary results suggest that ectopic expression of c-Myc in WEHI-231 cells overcomes the G2/M arrest induced by the JNK inhibitor.
Egr-1, an immediate early gene is overexpressed in our mouse model of BKS-2 B lymphoma. Inhibition of Egr-1 either with the antisense oligonucleotides specific for Egr-1 or with a retrovirus construct that expresses a repressor of Egr-1 (WT-Egr1) inhibits the basal proliferation of BKS-2 (M.G., J.K., and S.B., manuscript in preparation). Moreover, the Egr-1 promoter has an activator protein 1 (AP-1) binding site, suggesting a possible role for JNK signaling during activation of Egr-1 expression.70 Accordingly, we show that Egr-1 protein is reduced early in SP600125- or JNK siRNA-treated cells and is completely abrogated by 24 hours of treatment. Moreover, ectopic expression of Egr-1 partially rescued BKS-2 B-lymphoma cells from apoptosis induced by the JNK inhibitor SP600125. These data for the first time demonstrate that Egr-1 may be one of the survival factors downstream of JNK signaling. It was shown recently that in prostate cancer cells induction of p300/cyclic adenosine monophosphate response element binding protein (CBP) by Egr1 results in acetylation and stabilization of Egr1 and subsequent transactivation of survival genes.71
The prosurvival role for JNK in B-lymphoma growth was further substantiated by our in vivo studies in a mouse model of B lymphoma. In summary, our data demonstrate for the first time that the proliferation of primary B cells and B-lymphoma cells is dependent on JNK activation. Patients with activated B-cell-like DLBCLs have poor prognosis, with only 40% responding to chemotherapy.26 Our findings suggest that targeting JNK may have some important therapeutic implications in the treatment of B lymphomas.72
Prepublished online as Blood First Edition Paper, May 12, 2005; DOI 10.1182/blood-2004-10-3819.
Supported by National Institutes of Health (NIH) grants AI 21490, AG 05731, and CA 92372 to S. B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Our thanks are due to Drs E. C. Snow and Vivek Rangnekar for their critical comments on the manuscript and Jennifer Strange and Greg Bauman for flow cytometry. We thank Dr J. Yannelli for RAJI cells; Drs Richard May and Petr Starostik for OCI-Ly7 and OCI-Ly10 cells; Dr Gabriel Nunez for WEHI-231 Bcl-xL cells; and Dr John Monroe for pBX-EGR.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal