Abstract
OX40 and 4-1BB are members of the tumor necrosis factor (TNF) family of costimulatory receptors whose signaling is important for differential immune responses mediated by CD4+ or CD8+ T cells. Although activated T cells may acquire OX40/4-1BB double-positive phenotype and signaling from each receptor is expected to influence cell functions, the relevance between OX40 and 4-1BB has never been investigated before. While we were investigating the expression of OX40 and 4-1BB on activated human T cells, we found that they colocalize. The study of receptor gene–transfected cells showed that both receptors coendocytose and the complex of OX40 and 4-1BB was detected by specific ligands or antibodies (Abs). The heterodimer of OX40 and 4-1BB was identified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under nonreduced conditions and was associated with the tumor receptor–associated factor (TRAF) family proteins in a unique manner. Furthermore, the stimulation of OX40/4-1BB rendered cells sensitive to apoptosis induced by TNF-α that accompanied reduced activation of nuclear factor-κB (NF-κB). Finally, the OX40/4-1BB stimulation repressed the mitogen response in activated CD25+CD4+ T cells and preactivated CD8+ T cells. Thus, the OX40/4-1BB heterodimer appears to represent a unique regulatory receptor in activated T cells.
Introduction
Costimulatory receptor signaling is a necessary element for antigen-specific T-cell immune response by regulating proliferation, differentiation, and apoptosis.1-3 Studies demonstrated that the repertoire of costimulatory receptors on T cells increases following the primary activation via antigen-specific receptor and CD28 costimulatory receptor.1-3 OX40 (CD134) and 4-1BB (CD137) belong to the tumor necrosis factor (TNF) receptor (TNFR) family and represent T-cell costimulatory receptors expressed on cell activation.1-3 In several immunologic conditions such as viral infection, contact hypersensitivity, and protein-antigenic challenge, the signal resulting from the ligation of OX40 with OX40 ligand (OX40L) expressed on antigen-presenting cells (APCs) facilitates the generation of CD4+ memory T cells.4-7 The OX40/OX40L signal also regulates Th1 and Th2 responses in vivo in various Th1-specific or Th2-specific disease models.7-15 The 4-1BB/4-1BBL–mediated signaling has been shown to play a role in T-cell proliferation, activation-induced cell death prevention, promotion of the rejection of cardiac and skin allografts, eradication of established tumors, enhancement of integrin-mediated cell adherence, and increase of T-cell cytolytic potential.16-18 In support with the accumulating evidence indicating that OX40 signal and 4-1BB signal play different roles in T-cell immune response, it has been demonstrated that the intracellular signaling mechanism of OX40 differs from 4-1BB signaling.19 Consequently, T cells expressing both OX40 and 4-1BB would be influenced by the interplay of concomitant signals on cognate interaction with OX40L+4-1BBL+ APCs. It is, however, virtually unknown how such redundancy of TNFR family costimulatory receptors regulates antigen-induced T-cell responses.
We have investigated T cells simultaneously expressing OX40 and 4-1BB to understand how a concomitant expression of different costimulatory receptors modulates T-cell function. We found an evident cell-surface colocalization of OX40 and 4-1BB on OX40/4-1BB double-positive T cells. OX40 and 4-1BB were found to coendocytose in an antibody stimulation response against 4-1BB. The immunoprecipitation/Western blotting study demonstrated OX40/4-1BB heterodimers in gene-transfected cells and mitogen-induced T-cell blasts. Both OX40L and 4-1BBL bound the OX40/4-1BB heterodimer receptor. The association of tumor receptor–associated factor (TRAF) family proteins with the OX40/4-1BB heterodimer receptor was different from that of OX40 or 4-1BB receptors. Notably, the simultaneous stimulation of OX40 and 4-1BB rendered cells more sensitive to apoptosis induced by TNF-α, which was accompanied by reduced nuclear factor-κB (NF-κB) activation. The proliferative response of preactivated CD25+CD4+ T cells and preactivated CD8+ T cells, which comprised cells expressing both OX40 and 4-1BB, was repressed by the simultaneous ligation with OX40L and 4-1BBL. Accordingly, the expression of OX40 and 4-1BB in the same cell enabled T cells to acquire the OX40/4-1BB heterodimer receptors, which could have de novo regulatory functions. This study unveils a novel form of receptor, in which 2 distinct TNFR family members working together regulate the function of preactivated human T cells.
Materials and methods
Construction of expression vectors
Human OX40, 4-1BB, and CD40L receptors were obtained by reverse transcription–polymerase chain reaction (RT-PCR) amplification from a human T-cell blast total RNA library, followed by subcloning into a mammalian expression vector, pCDNA3 (Invitrogen, Huntsville, AL). OX40 was subcloned into a green fluorescent protein (GFP) expression vector, pEGFP-N1 (Clontech, Palo Alto, CA), to couple its cytoplasmic tail with GFP. 4-1BB and CD40L were subcloned into a red fluorescent protein (RFP) expression vector, pDsRed1-N1 (Clontech), to modify the cytoplasmic tail with RFP. HA-tagged, full-length OX40 and FLAG-tagged, full-length 4-1BB were subcloned into pCDNA3. Glutathione-S-transferase (GST) fusion proteins containing the extracellular domains of OX40L and 4-1BBL were prepared using cDNA fragments obtained by RT-PCR amplification from human monocyte cell line THP-1 total RNA and subcloned in-frame with the GST moiety into pGEX expression vector (Amersham Pharmacia, Piscataway, NJ). The expression vectors encoding pCMV-HA-TRAF1, -TRAF2, -TRAF3, -TRAF4, and -TRAF5 have been described previously.20
Laser confocal microscopic study and flow cytometric analysis
To examine distribution of OX40 and 4-1BB in T cells and transfectants, human peripheral blood (PBL) T cells stimulated with anti-CD3 (1 μg/mL) and anti-CD28 (1 μg/mL) antibodies (Abs) for 3 days were stained with fluorescein isothiocyanate (FITC)–labeled anti-OX40 (Becton Dickinson, San Jose, CA) and phycoerythrin (PE)–labeled anti–4-1BB (Becton Dickinson) or FITC-labeled anti-CD27 Ab (Becton Dickinson). HEK293 cells cotransfected with OX40-GFP and 4-1BB-RFP or OX40-GFP and CD40L-RFP, as a control, were subsequently incubated overnight at 37°C prior to confocal microscopic analysis. The cells were monitored by laser scanning microscope (LSM 510, Carl Zeiss, Toronto, ON, Canada) with 63 × 1.3 NA oil immersion lens and LSM software (Carl Zeiss) for the distribution of OX40 and 4-1BB. The cell-surface OX40/4-1BB expression of CD4+ T cells and CD8+ T cells stimulated with anti-CD3 and anti-CD28 Abs or nonstimulated cells was determined by 3-color flow cytometry using anti-OX40–FITC, anti–4-1BB–PE-cyanin 5 (Cy5) Abs (PharMingen, Mississauga, ON, Canada), and CD4-PE or CD8-PE Abs (PharMingen) using a fluorescence-activated cell sorting FACScan (Becton Dickinson).
Endocytosis analysis
HEK293 transfectants expressing OX40-GFP and 4-1BB–RFP were incubated with 1 μg/mL anti–4-1BB Ab (AmGen, Seattle, WA) at 4°C for 1 hour, 37°C for 30 minutes, or 37°C in complete culture medium containing 0.45 M sucrose for 30 minutes. Cells were then washed twice with ice-cold phosphate-buffered saline (PBS), fixed in PBS containing 1% paraformaldehyde, and analyzed by a laser scanning confocal microscope (LSM 510, Carl Zeiss).
Coimmunoprecipitation and immunoblotting
One percent Nonidet P-40 lysis buffer21 cell extracts (107 cells/mL) were derived from HEK293 cells transfected with HA-tagged OX40 and FLAG-tagged 4-1BB, or human PBL T-cell blasts. Aliquots of 1 mL of clear lysates were incubated for 1 hour with agitation at 4°C by the use of Abs specific for HA (mouse anti-HA [F-7, Santa Cruz Biotechnology, Santa Cruz, CA]), FLAG (mouse anti-FLAG [M2, Sigma, St Louis, MO], mouse anti-DYKDDDK (2EL-1B11, Chemicon, Temecula, CA]), OX40 (rabbit anti-OX40 [Santa Cruz Biotechnology]), 4-1BB (rabbit anti–4-1BB [Chemicon]), or control Abs as indicated in the figures. Then, 30 μL protein A/G-Sepharose beads (Santa Cruz Biotechnology) were washed with lysis buffer and were added and further incubated at 4°C for 1 hour. The beads were then washed 3 times in the cold lysis buffer and proteins were eluted by incubating at 70°C for 10 minutes in NuPAGE sample buffer (Invitrogen), separated by 10% NuPAGE in MOPS (3-[N-Morpholino]propanesulphonic acid) buffer (Invitrogen). Protein blots were transferred to polyvinylidene difluoride (PVDF) membranes (Pall, East Hills, NY) for immunoblotting. The membranes were incubated with Abs specific for HA (horseradish peroxidase [HRP]–rat anti-HA [3F10, Roche, Indianapolis, IN]), FLAG (mouse anti-FLAG [M2, Sigma]), HRP-chicken anti-FLAG (Immunology Consultants Laboratories, Newberg, OR), OX40 (rabbit anti-OX40 [Santa Cruz Biotechnology]), and 4-1BB (rabbit anti–4-1BB [Chemicon]) as indicated in the figures. The HRP-conjugated detection Abs (HRP-anti–mouse IgG, HRP-anti–rabbit IgG, Pierce, Rockford, IL) were applied when necessary. Protein blots were visualized by the enhanced chemiluminescence (ECL) detection system (Amersham Life Science, Piscataway, NJ). In some experiments, membranes were reprobed with different Abs.
Ligand-receptor binding analysis
Cell lysates prepared from HEK293 cells transfected with OX40-HA, 4-1BB–FLAG, or OX40-HA/4-1BB–FLAG were mixed with GST-OX40L, GST–4-1BBL, or GST only Sepharose beads. Proteins bound to GST-OX40L, GST–4-1BBL, or GST were analyzed by 10% NuPAGE in MOPS buffer (Invitrogen) and subjected to immunoblotting with anti-HA or anti-FLAG Abs.
Apoptosis assay
Apoptosis was analyzed by annexin binding and poly(ADP-ribose)polymerase (PARP) cleavage assays. HEK293 transfectants were treated with recombinant human TNF-α 200 U/mL (Roche) or medium control for 15 hours. Cells were harvested and stained with annexin V (Becton Dickinson)22 ; the staining profile was assessed using FACScan. In parallel experiments, the same cells were subjected for the analysis of PARP cleavage by Western blotting as reported before.23,24
NF-κB reporter gene analysis
Six-well plates were seeded with HEK293 cells at a density of 5 × 105 cells/well in 2 mL medium. Triplicate wells were transfected with 0.3 μg NF-κB luciferase reporter plasmid (κB-Luc)25 and 1 μg plasmid harboring OX40 or 4-1BB by LipofectAMINE reagent (Invitrogen). Transfectants were maintained at 37°C for 16 hours and stimulated with anti–human OX40 (Santa Cruz Biotechnology), anti–human 4-1BB (Amgen), or control Abs for 6 hours. Cells were harvested and luciferase reporter gene activity assayed as reported previously.26 In general, more than 60% of cells were transfected with the genes of interest by the method we used.
Studies using mitogen-induced CD25+CD4+ cells and mitogen-stimulated CD8+ cells
To prepare mitogen-induced CD4+CD25+ cells, CD4+ human PBL T cells were stimulated with anti-CD3 Ab (1 μg/mL) plus anti-CD28 Ab (1 μg/mL) for 3 days. CD25+ cells were purified by anti-CD25 (mouse IgG), PE-anti–mouse IgG, then positively selected by a PE-specific purification kit (StemCell Technologies, Vancouver, BC, Canada). CD4+CD25+ cells constituted more than 90% of the cells purified as determined by flow cytometry. The cells were then stimulated by anti-CD3 Ab (1 μg/mL), anti-CD28 Ab (1 μg/mL), and 50 U/mL recombinant human interleukin 2 (IL-2; Sigma) in the presence of soluble ligand fusion proteins for 3 days before [3H]thymidine incorporation assays.
To prepare preactivated CD8+ T cells, CD8+ T cells were enriched from PBL using CD8 T cell-specific enriching reagent (RosetteSep, StemCell Technologies). As determined by flow cytometry, CD8+ cells were purified to the extent of more than 80%. These cells were stimulated with anti-CD3 Ab (1 μg/mL) plus anti-CD28 Ab (1 μg/mL) for 3 days. The cells were further cultured in the medium supplemented by anti-CD3 Ab (1 μg/mL) and 50 U/mL recombinant human IL-2 in the presence of soluble ligand fusion proteins for 3 days before [3H]thymidine incorporation assays.
Results
OX40 and 4-1BB colocalize on human PBL T cells
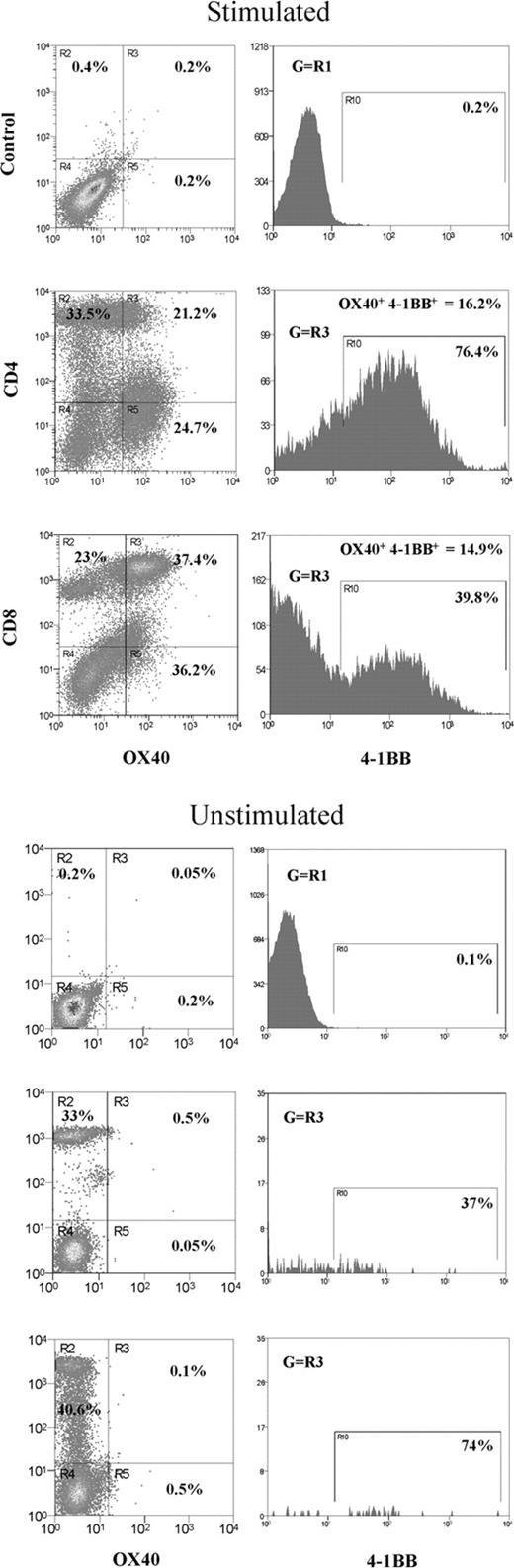
To obtain an insight into the expression profile of OX40 and 4-1BB in CD4+ or CD8+ T-cell subsets, mitogen-stimulated human PBL T cells were triple-color stained with specific Abs and analyzed by flow cytometry (Figure 1). OX40 only or 4-1BB only expressing CD4+ cells shared 21% and 25% of total CD4+ T cells, respectively, whereas OX40 only or 4-1BB only expressing CD8+ cells shared 37% and 18%. Along with OX40+ or 4-1BB+ cells, OX40+4-1BB+ double-positive cells were also observed. The study showed about 16% of CD4+ and about 15% of CD8+ cells expressed both OX40 and 4-1BB. Unexpectedly, confocal microscopy showed that OX40 and 4-1BB colocalize in OX40+4-1BB+ cells (Figure 2A). The controls, including CD27, CD40L, and CD28, did not overlap with OX40 or 4-1BB (only CD27 data were shown in Figure 2A). A similar observation was made in HEK293 cells transfected with OX40 and 4-1BB and stained on the cell surface with anti-OX40 Ab and anti–4-1BB Ab (data not shown). The results prompted us to hypothesize that OX40 and 4-1BB associate in activated T cells in a fashion similar to what was previously observed for a CD28 splice variant (CD28i) and CD28 or CD40L.26,28
To investigate a possible complex formation between OX40 and 4-1BB in detail, HEK293 cells were transfected with a GFP-tagged OX40 and an RFP-tagged 4-1BB (Figure 2B). The confocal microscopy of those double-transfected cells further revealed that the distribution of OX40 and 4-1BB greatly overlapped on the cell surface. In contrast with 4-1BB, the CD40L-RFP distribution did not overlap with OX40-GFP. Significantly, the cross-linking of 4-1BB by the specific Ab resulted in the clathrin-dependent coendocytosis of 4-1BB along with OX40 (Figure 2C), suggesting a functional association of both receptors.
The complex of OX40 and 4-1BB is extracted by specific Abs or ligand-mediated precipitation
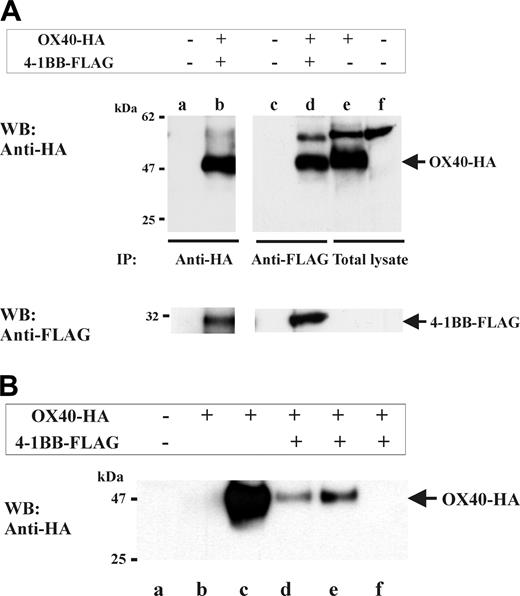
To further investigate the OX40/4-1BB complex, we performed immunoprecipitation studies using OX40/4-1BB double transfectant cell extracts. Specifically, HA-tagged OX40 and FLAG-tagged 4-1BB were transfected into HEK293 cells. Next, 1% NP-40 cell extracts were prepared and subjected to 4-1BB-specific or OX40-specific immunoprecipitation (Figure 3A). Western blotting specific for OX40 (by anti-HA Ab) or for 4-1BB (by anti-FLAG Ab), indeed, demonstrated the presence of OX40/4-1BB complex in the immunoprecipitates. Moreover, a similar experiment using soluble ligands to OX40 or 4-1BB, in place of specific Abs, also demonstrated that both OX40L and 4-1BBL can bind to the OX40/4-1BB complex (Figure 3B).
Detection of OX40 and 4-1BB heterodimer by nonreduced SDS-PAGE
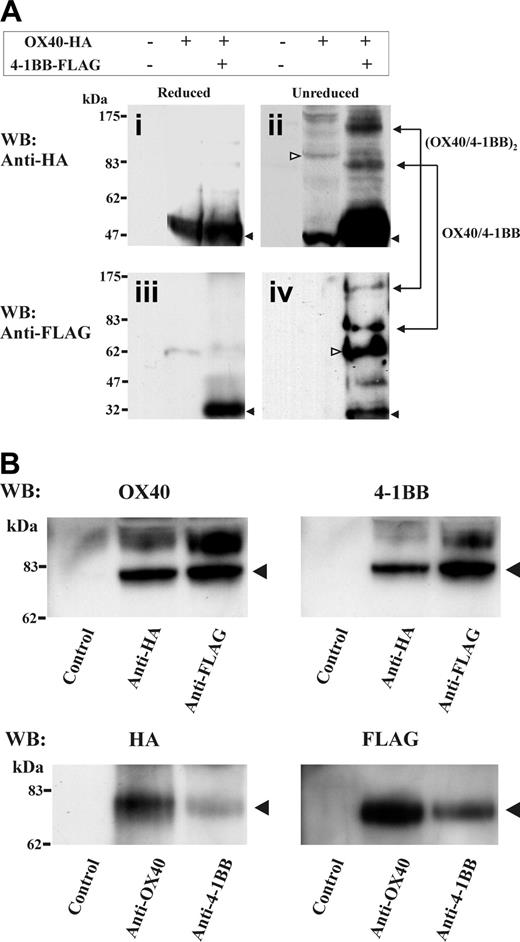
It was surprising to observe that OX40 and 4-1BB, both largely different in the ectodomain and cytoplasmic tail domains, form a tight complex stable in 1% NP-40 lysis buffer. To investigate how OX40 and 4-1BB complex, we performed an analysis of double-transfected HEK cell extracts by SDS-PAGE under a nonreduced condition. Significantly, we observed proteins at about 80 kDa and about 160 kDa in total cell lysates under a nonreduced condition by HA- or FLAG-specific Western blotting (Figure 4A). These molecular masses likely represent a heterodimer of OX40 (∼48 kDa) and 4-1BB (∼30 kDa) and a possible dimer of the heterodimers. To further investigate the probable heterodimer of about 80 kDa, the OX40-HA and 4-1BB-FLAG double-transfected cell lysates were immunoprecipitated with anti-HA Ab or anti-FLAG Ab, then analyzed by OX40-specific or 4-1BB-specific Western blotting under a nonreduced condition. Alternatively, the immunoprecipitates were prepared using anti-OX40 Ab and anti–4-1BB Ab, then analyzed by HA-specific or FLAG-specific Western blotting. The study demonstrated the presence of the protein bands at about 80 kDa that were reactive with both OX40-HA-specific and 4-1BB-FLAG-specific Abs (Figure 4B). The data suggest that OX40/4-1BB complexes as a heterodimer in the HEK293 double transfectants.
Induction of OX40+4-1BB+ cells in CD3- and CD28-stimulated PBL T cells. In the left panel, total PBL was stimulated with anti-CD3 Ab (OKT3; 1 μg/mL) and anti-CD28 Ab (1 μg/mL) for 3 days before 3-color flow cytometry specific for OX40, 4-1BB, and CD4 or CD8. Right panels show the data for PBL cells without mitogenic stimulation. R3 quadrants in the dot plot indicate cells expressing OX40 gated for CD4+ or CD8+ T cells. Histograms indicate cells positive for 4-1BB gated in quadrant R3. The proportion of OX40+4-1BB+ subsets gated for stimulated CD4+ or CD8+ T cells is indicated in the top portion of the histograms.
Induction of OX40+4-1BB+ cells in CD3- and CD28-stimulated PBL T cells. In the left panel, total PBL was stimulated with anti-CD3 Ab (OKT3; 1 μg/mL) and anti-CD28 Ab (1 μg/mL) for 3 days before 3-color flow cytometry specific for OX40, 4-1BB, and CD4 or CD8. Right panels show the data for PBL cells without mitogenic stimulation. R3 quadrants in the dot plot indicate cells expressing OX40 gated for CD4+ or CD8+ T cells. Histograms indicate cells positive for 4-1BB gated in quadrant R3. The proportion of OX40+4-1BB+ subsets gated for stimulated CD4+ or CD8+ T cells is indicated in the top portion of the histograms.
Confocal study of OX40 and 4-1BB on activated T cells and transfectant cells. (A) OX40 and 4-1BB colocalize on activated T cells. Mitogen-stimulated total T cells were stained for OX40 (green), 4-1BB (red), or CD27 (red) and analyzed by confocal microscopy. In the top 3 panels, stimulated PBLs were stained with OX40 and 4-1BB. In the right end panel, green and red fluorescence overlapped and cells exhibiting colocalization of OX40 and 4-1BB are indicated by arrows. In Ai-ii, cells positive for OX40 and 4-1BB staining (i) and cells positive for OX40 and CD27 (negative control) staining (ii) were analyzed at higher magnification for colocalization. (B) OX40 and 4-1BB colocalize in gene-transfected HEK293 cells. HEK293 cells were gene-transfected with OX40-GFP and 4-1BB-RFP or CD40L-RFP for 16 hours before confocal microscopy. In the right end panels, green and red fluorescence were overlapped to show colocalization of receptors. (C) OX40 and 4-1BB coendocytose following cross-linking to 4-1BB. HEK293 cells were gene-transfected with OX40-GFP and 4-1BB–RFP for 16 hours. Cells were treated for 30 minutes with anti–4-1BB Ab (1 μg/mL) at 4°C, 37°C, or at 37°C in the presence of sucrose (0.45 M), which prevented clathrin-dependent endocytosis.27 In the right end panels, green and red fluorescences were overlapped to emphasize the coendocytosis of receptors.
Confocal study of OX40 and 4-1BB on activated T cells and transfectant cells. (A) OX40 and 4-1BB colocalize on activated T cells. Mitogen-stimulated total T cells were stained for OX40 (green), 4-1BB (red), or CD27 (red) and analyzed by confocal microscopy. In the top 3 panels, stimulated PBLs were stained with OX40 and 4-1BB. In the right end panel, green and red fluorescence overlapped and cells exhibiting colocalization of OX40 and 4-1BB are indicated by arrows. In Ai-ii, cells positive for OX40 and 4-1BB staining (i) and cells positive for OX40 and CD27 (negative control) staining (ii) were analyzed at higher magnification for colocalization. (B) OX40 and 4-1BB colocalize in gene-transfected HEK293 cells. HEK293 cells were gene-transfected with OX40-GFP and 4-1BB-RFP or CD40L-RFP for 16 hours before confocal microscopy. In the right end panels, green and red fluorescence were overlapped to show colocalization of receptors. (C) OX40 and 4-1BB coendocytose following cross-linking to 4-1BB. HEK293 cells were gene-transfected with OX40-GFP and 4-1BB–RFP for 16 hours. Cells were treated for 30 minutes with anti–4-1BB Ab (1 μg/mL) at 4°C, 37°C, or at 37°C in the presence of sucrose (0.45 M), which prevented clathrin-dependent endocytosis.27 In the right end panels, green and red fluorescences were overlapped to emphasize the coendocytosis of receptors.
Heterocomplex formation between OX40 and 4-1BB in gene-transfectant cells and T-cell blasts. (A) OX40 and 4-1BB coimmunoprecipitate from cell extracts derived from HEK293 transfectants. HEK293 cells were transfected with OX40-HA (full-length HA-tagged OX40) and 4-1BB-FLAG (full-length FLAG-tagged 4-1BB) as indicated on the top of the figure. One percent NP-40 cell extracts were immunoprecipitated with anti-FLAG (for 4-1BB immunoprecipitation) or anti-HA (for OX40 immunoprecipitation), then analyzed by HA-specific or FLAG-specific Western blotting as indicated. Lanes e-f show total cell lysates of OX40-HA–transfected HEK293 or nontransfected HEK293. Positions of OX40-HA and 4-1BB–FLAG are indicated by arrows on the right. (B) OX40 and 4-1BB coprecipitate by OX40L-GST or 4-1BB–GST from cell extracts of gene-transfectant cells. HEK293 cells were transfected with OX40-HA and 4-1BB–FLAG as indicated on the top of the figure. One percent NP-40 cell extracts were subjected to OX40-GST–or 4-1BB-GST–specific precipitation then analyzed by HA-specific Western blotting (WB). Lane a, precipitated by GST beads; lane b, precipitated by 4-1BBL–GST; lane c, precipitated by OX40L-GST; lane d, precipitated by 4-1BBL–GST; lane e, precipitated by OX40L-GST; and lane f, precipitated by GST. Position of OX40-HA is indicated by the arrow on the right.
Heterocomplex formation between OX40 and 4-1BB in gene-transfectant cells and T-cell blasts. (A) OX40 and 4-1BB coimmunoprecipitate from cell extracts derived from HEK293 transfectants. HEK293 cells were transfected with OX40-HA (full-length HA-tagged OX40) and 4-1BB-FLAG (full-length FLAG-tagged 4-1BB) as indicated on the top of the figure. One percent NP-40 cell extracts were immunoprecipitated with anti-FLAG (for 4-1BB immunoprecipitation) or anti-HA (for OX40 immunoprecipitation), then analyzed by HA-specific or FLAG-specific Western blotting as indicated. Lanes e-f show total cell lysates of OX40-HA–transfected HEK293 or nontransfected HEK293. Positions of OX40-HA and 4-1BB–FLAG are indicated by arrows on the right. (B) OX40 and 4-1BB coprecipitate by OX40L-GST or 4-1BB–GST from cell extracts of gene-transfectant cells. HEK293 cells were transfected with OX40-HA and 4-1BB–FLAG as indicated on the top of the figure. One percent NP-40 cell extracts were subjected to OX40-GST–or 4-1BB-GST–specific precipitation then analyzed by HA-specific Western blotting (WB). Lane a, precipitated by GST beads; lane b, precipitated by 4-1BBL–GST; lane c, precipitated by OX40L-GST; lane d, precipitated by 4-1BBL–GST; lane e, precipitated by OX40L-GST; and lane f, precipitated by GST. Position of OX40-HA is indicated by the arrow on the right.
Presence of the OX40 and 4-1BB heteromolecular complex in PBL T-cell blasts
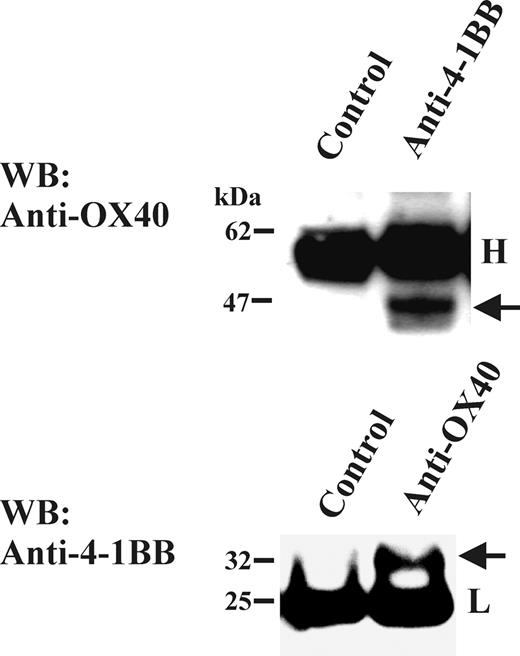
To investigate the association of OX40 and 4-1BB in more physiologically relevant setting, we tested human PBLs for the presence of the OX40/4-1BB complex. The complex of OX40 and 4-1BB was readily detected in the mitogen-activated PBL T-cell lysates by coimmunoprecipitation/Western blotting studies (Figure 5). In control experiments, CD27 was not detected in immunoprecipitates specific for either OX40 or 4-1BB (data not shown). Thus, the OX40/4-1BB complex seemed to be a result of a specific interaction. Collectively, the study demonstrated that pairing between OX40 and 4-1BB occurs normally in mitogen-induced primary T-cell blasts.
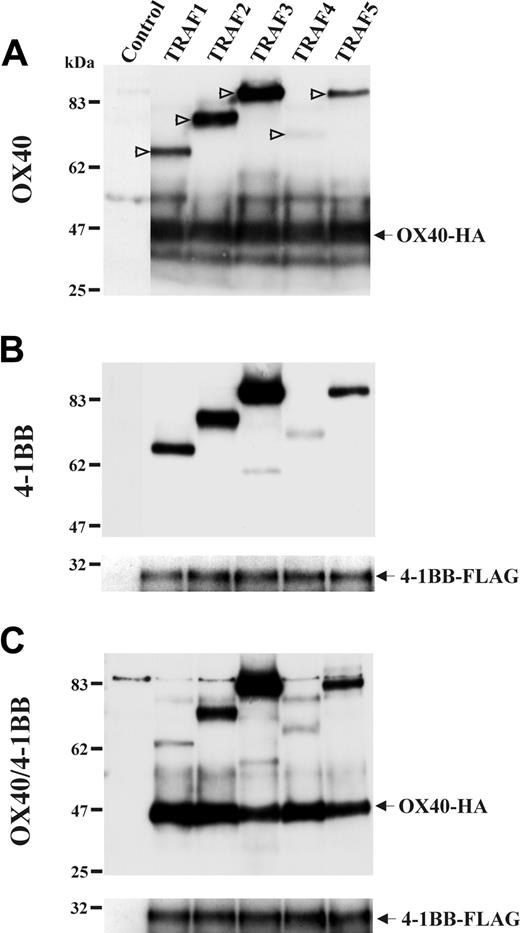
The OX40/4-1BB heterodimer weakly associates with TRAF1
On identifying OX40/4-1BB heterodimer in activated T cells, we had a unique opportunity to investigate the function of this receptor of a novel form. We investigated an association of TRAF proteins with the OX40/4-1BB heterodimer receptor because OX40 and 4-1BB signaling mechanisms were characterized relevant to the TRAF family protein association in previous studies.5,29-31 A study involving 5 members of TRAF protein family (TRAF1-TRAF5)18 demonstrated that OX40 and 4-1BB associated with TRAF proteins in a similar fashion. Interestingly, the OX40/4-1BB heterodimer showed a weaker association with TRAF1 than that observed for OX40 or 4-1BB alone (Figure 6). The data suggested that the signaling triggered by OX40/4-1BB heterodimer is different from that triggered by OX40 or 4-1BB only.
Detection of OX40 and 4-1BB heterodimer by SDS-PAGE under a nonreduced condition. (A) HEK293 cells were transfected with OX40-HA and 4-1BB–FLAG as indicated on the top of figure. One percent NP-40 cell extracts were characterized by HA- or FLAG-specific Western blotting under reduced or nonreduced conditions. In subpanels i-ii, monomer of OX40 (∼48 kDa) is indicated by filled arrows. In subpanels ii and iv, presumed dimer of OX40 (∼96 kDa) and dimer of 4-1BB (∼60 kDa) are indicated by open arrows. Blots for presumed heterodimer (∼78 kDa) and the dimer of heterodimer (∼160 kDa) were observed in both HA-specific and FLAG-specific Western blots and indicated at right side in subpanels ii and iv. (B) The OX40-HA and 4-1BB–FLAG double-transfectant HEK cell extracts were immunoprecipitated with Abs specific for HA, FLAG, OX40, 4-1BB, or isotype control (indicated below panels). Immunoprecipitates were characterized by OX40, 4-1BB, HA-tag–or FLAG-tag–specific Western blotting under nonreduced conditions. Blots for presumed heterodimer (∼78 kDa) were observed in both OX40-HA–specific and 4-1BB-FLAG–specific Western blotting and indicated at the right side of each panel.
Detection of OX40 and 4-1BB heterodimer by SDS-PAGE under a nonreduced condition. (A) HEK293 cells were transfected with OX40-HA and 4-1BB–FLAG as indicated on the top of figure. One percent NP-40 cell extracts were characterized by HA- or FLAG-specific Western blotting under reduced or nonreduced conditions. In subpanels i-ii, monomer of OX40 (∼48 kDa) is indicated by filled arrows. In subpanels ii and iv, presumed dimer of OX40 (∼96 kDa) and dimer of 4-1BB (∼60 kDa) are indicated by open arrows. Blots for presumed heterodimer (∼78 kDa) and the dimer of heterodimer (∼160 kDa) were observed in both HA-specific and FLAG-specific Western blots and indicated at right side in subpanels ii and iv. (B) The OX40-HA and 4-1BB–FLAG double-transfectant HEK cell extracts were immunoprecipitated with Abs specific for HA, FLAG, OX40, 4-1BB, or isotype control (indicated below panels). Immunoprecipitates were characterized by OX40, 4-1BB, HA-tag–or FLAG-tag–specific Western blotting under nonreduced conditions. Blots for presumed heterodimer (∼78 kDa) were observed in both OX40-HA–specific and 4-1BB-FLAG–specific Western blotting and indicated at the right side of each panel.
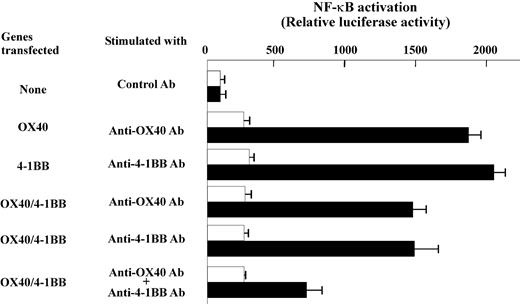
Reduced induction of NF-κB activation and cell survival in transfectant cells following OX40/4-1BB stimulation
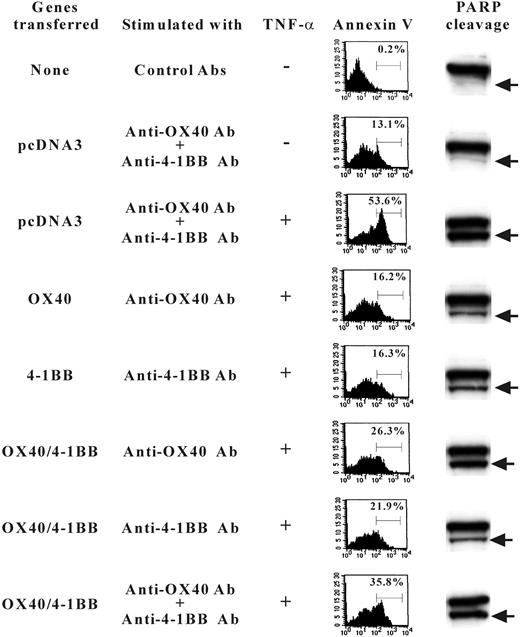
Previously, the signaling mechanism by OX40 and 4-1BB was characterized relevant to NF-κB activation pathways along with TRAF family protein association.31,32 We, therefore, extended our study by investigating the NF-κB signaling in gene transfectant cells. The cells transfected with OX40 alone or 4-1BB alone showed prominent induction of NF-κB activity when cells were stimulated with anti-OX40 Ab or anti–4-1BB Ab, whereas the double-transfected cells showed evidently lower NF-κB activation when stimulated with both anti-OX40 and anti–4-1BB Abs (Figure 7). Similar results were observed when soluble OX40L or 4-1BBL fusion proteins were used to stimulate cells (data not shown). The data may be construed that the OX40/4-1BB heterodimer receptor poorly couples to the NF-κB pathway or inhibits the activation of NF-κB. The data prompted us to test whether the signaling induced by concomitant stimulation against OX40/4-1BB heterodimer receptor alters the cell death induced by TNF-α in transfectant cells. As shown in Figure 8, the stimulation by OX40 or 4-1BB alone increased the cell survival after TNF-α exposure because annexin V+ cells are about 16% compared with about 53.6% of TNF-α–treated control cells. On the contrary, the concurrent expression of OX40 and 4-1BB and the concomitant stimulation of each receptor resulted in a poor protection against TNF-α–induced cell death because annexin V+ cells increased to 35.8%. Consistently, the assays for the PARP cleavage fragments resulting from caspase-3/caspase-7–mediated proteolysis in cell lysates from experimental groups demonstrated that the activity of caspase-3/caspase-7 correlates with the staining levels of annexin V (Figure 8). The data showed that the concomitant stimulation of OX40 and 4-1BB results in a repressed cell survival when compared to cells stimulated with OX40 alone or 4-1BB alone. The data indicate that the signaling by the OX40/4-1BB heterodimer receptor renders cells prone to apoptosis via NF-κB pathway inhibition.
Presence of OX40/4-1BB complex in cell lysate of PBL T-cell blasts. Total T cells were stimulated with anti-CD3 Ab (OKT3; 1 μg/mL) and anti-CD28 Ab (1 μg/mL) for 3 days. One percent NP-40 cell extracts were immunoprecipitated with anti-OX40 Ab or anti-4-1BB Ab, then analyzed by OX40-specific or 4-1BB-specific Western blotting in a reduced condition. Immunoprecipitation Abs or control Ab is indicated on the top of each panel. H indicates the position of immunoglobulin heavy chain; L, position of immunoglobulin light chain. The arrow in the top panel indicates the position of OX40 and the arrow in the bottom panel indicates the position of 4-1BB.
Presence of OX40/4-1BB complex in cell lysate of PBL T-cell blasts. Total T cells were stimulated with anti-CD3 Ab (OKT3; 1 μg/mL) and anti-CD28 Ab (1 μg/mL) for 3 days. One percent NP-40 cell extracts were immunoprecipitated with anti-OX40 Ab or anti-4-1BB Ab, then analyzed by OX40-specific or 4-1BB-specific Western blotting in a reduced condition. Immunoprecipitation Abs or control Ab is indicated on the top of each panel. H indicates the position of immunoglobulin heavy chain; L, position of immunoglobulin light chain. The arrow in the top panel indicates the position of OX40 and the arrow in the bottom panel indicates the position of 4-1BB.
The association of OX40/4-1BB heterodimer with TRAF proteins. HEK293 cells were transfected with HA-tagged TRAF1, 2, 3, 4, or 5 for 16 hours as indicated in the top of each panel. Cells were concomitantly transfected with OX40-HA and 4-1BB–FLAG or both as indicated on the left of each panel. One percent NP-40 cell extracts were immunoprecipitated with anti-OX40 Ab in panel A and immunoprecipitated with anti–4-1BB Ab in panels B-C. Immunoprecipitates were analyzed in a reduced condition by SDS-PAGE and Western blotting specific for HA or FLAG. In panel A, positions of TRAF proteins are indicated by open arrows.
The association of OX40/4-1BB heterodimer with TRAF proteins. HEK293 cells were transfected with HA-tagged TRAF1, 2, 3, 4, or 5 for 16 hours as indicated in the top of each panel. Cells were concomitantly transfected with OX40-HA and 4-1BB–FLAG or both as indicated on the left of each panel. One percent NP-40 cell extracts were immunoprecipitated with anti-OX40 Ab in panel A and immunoprecipitated with anti–4-1BB Ab in panels B-C. Immunoprecipitates were analyzed in a reduced condition by SDS-PAGE and Western blotting specific for HA or FLAG. In panel A, positions of TRAF proteins are indicated by open arrows.
OX40/4-1BB signaling represses proliferation in preactivated CD25+CD4+ T cells and preactivated CD8+ T cells
In an attempt to determine the function of the signaling by the OX40/4-1BB heterodimer, we investigated the preactivated CD25+CD4+ T-cell subset and CD8+ T cells. It has been recently demonstrated that CD25+CD4+ regulatory T cells are positive for both OX40 and 4-1BB costimulatory receptors.33 We also found the expression of OX40 and 4-1BB in preactivated CD25+CD4+ T cells (data not shown). Alternatively, in our study about 15% of CD8+ cells also turned out to be OX40 and 4-1BB double positive after 3 days of mitogenic stimulation (Figure 1). Similar to what has been reported before,34 the addition of the mitogenic cocktail made of anti-CD3 Ab, anti-CD28 Ab, and IL-2 into the culture induces DNA synthesis in preactivated CD25+CD4+ T cells (Figure 9A). The simultaneous presence of OX40L and 4-1BBL in culture, however, significantly reduced DNA synthesis in preactivated CD25+CD4+ T cells. The influence of OX40L and 4-1BBL on the preactivated CD8+ T cells was similar to that seen for CD25+CD4+ T cells. The cell proliferation induced by anti-CD3 Ab and IL-2 in CD8+ cells was repressed when both ligands were added to the culture (Figure 9B). In the other experimental groups, neither OX40L alone nor 4-1BBL promoted a notable increase of DNA synthesis in preactivated CD25+CD4+ T cells and only a slight repression was observed. For preactivated CD8+ T cells, 4-1BBL augmented the DNA synthesis by about 20%, whereas OX40L did not change the response. Our data suggest that dual stimulation with OX40L and 4-1BBL triggers a negative regulatory effect on preactivated primary CD25+CD4+ T cells and on preactivated CD8+ T cells as a probable result of the OX40/4-1BB heterodimer receptor signaling.
Reduced induction of NF-κB activation by OX40/4-1BB double-transfectant cells stimulated with specific Abs. OX40-HA and 4-1BB–FLAG double-transfectant or nontransfectant HEK cells were stimulated by anti-OX40 (1 μg/mL), anti–4-1BB (1 μg/mL), or control Ab (1 μg/mL) as indicated. □, nonstimulated cells; ▪, cells stimulated by control or specific Abs. The value of NF-κB reporter gene only transfected cells (the top group of cells indicated as “None” in the left) was set as 100 to calculate relative luciferase activity. Each column indicates an average and SD of triplicate samples.
Reduced induction of NF-κB activation by OX40/4-1BB double-transfectant cells stimulated with specific Abs. OX40-HA and 4-1BB–FLAG double-transfectant or nontransfectant HEK cells were stimulated by anti-OX40 (1 μg/mL), anti–4-1BB (1 μg/mL), or control Ab (1 μg/mL) as indicated. □, nonstimulated cells; ▪, cells stimulated by control or specific Abs. The value of NF-κB reporter gene only transfected cells (the top group of cells indicated as “None” in the left) was set as 100 to calculate relative luciferase activity. Each column indicates an average and SD of triplicate samples.
TNF-α–induced apoptosis of 4-1BB/OX40-transfected cells. HEK293 cells were transfected with OX40-HA or 4-1BB–FLAG or both or control pcDNA3 as indicated on the left. Sixteen hours later cells were stimulated with TNF-α in the presence and absence of Abs against OX40 or 4-1BB. Fifteen hours later, cells were stained with FITC–annexin V to determine the proportion of cells undergoing apoptosis (indicated as percent in the panels of flow cytometry results). Alternatively, cell extracts were analyzed by SDS-PAGE for PARP-specific Western blotting that detects native PARP (115 kDa, shown as the top bands in panels on right) and bottom bands (89 kDa) indicated by arrows resulting from the caspase cleavages.
TNF-α–induced apoptosis of 4-1BB/OX40-transfected cells. HEK293 cells were transfected with OX40-HA or 4-1BB–FLAG or both or control pcDNA3 as indicated on the left. Sixteen hours later cells were stimulated with TNF-α in the presence and absence of Abs against OX40 or 4-1BB. Fifteen hours later, cells were stained with FITC–annexin V to determine the proportion of cells undergoing apoptosis (indicated as percent in the panels of flow cytometry results). Alternatively, cell extracts were analyzed by SDS-PAGE for PARP-specific Western blotting that detects native PARP (115 kDa, shown as the top bands in panels on right) and bottom bands (89 kDa) indicated by arrows resulting from the caspase cleavages.
Curiously, the proliferation of preactivated CD8+ T cells was consistently repressed by nearly 40% when cells were treated with both OX40L and 4-1BBL fusion proteins despite the fact that OX40/4-1BB double-positive cells represented only 15% of the total CD8+ T cells. The data suggest a regulatory effect by OX40/4-1BB double-positive cells against bystander cells, which is triggered by the dual ligation of OX40 and 4-1BB. The nature of regulatory signaling and the mechanism of possible bystander effects must be investigated.
Discussion
Our results demonstrate the presence of the OX40/4-1BB heterocomplex receptor in gene-transfected cells as well as in primary T cells. The data indicate that the OX40/4-1BB heterodimer receptor complexes with TRAF proteins differently than OX40 alone or 4-1BB alone. Furthermore, the heterodimer seems to trigger a signal, which represses cell growth and cell survival. Finding both receptors on only specific subsets of T cells, for example, CD25+CD4+ T cells, suggests a specific role of the heterodimer receptor. Studies of CD25+CD4+ regulatory T cells, a subset within CD25+CD4+ T cells, showed that these cells are susceptible to apoptosis and it is difficult to induce proliferation in response to mitogenic stimuli.35,36 Accordingly, the expression of both OX40 and 4-1BB on the same cell may cause negative effects in T cells. Indeed, despite exhausting efforts to establish stable gene transfectant cells expressing both OX40 and 4-1BB, no such transfectant line has been successfully generated in our laboratory. Because transfectant cells positive for OX40 or 4-1BB alone were established without any difficulty, it is likely that the expression of OX40/4-1BB heterodimer could cause a negative impact in recipient cells by inhibiting cell growth or by inducing cell death. We have also tried the gene transfection into HEK293 cells and Jurkat cells but neither cell line was stably transformed into an OX40+4-1BB+ cell line. Noteworthy, in the transient gene transfection experiments using Jurkat cells, OX40-GFP and 4-1BB-RFP showed a strong colocalization in double-transfectant cells, although these cells were often accompanied by abnormal balloonlike phenotypes (A.O., unpublished data, January 2004). Together, it is thus probable that acquiring both OX40 and 4-1BB may possess significant means in terminating cell replication potential and such effect is not specific to T cells.
Cell growth inhibition of CD25+CD4+ T cells and CD8+ T cells by soluble OX40L and 4-1BBL. (A) CD4+ T cells were stimulated with anti-CD3 Ab (1 μg/mL) and anti-CD28 Ab (1 μg/mL) for 4 days. CD25+ cells were positively purified by anti-CD25 Ab and cell separation kit (StemCell Technologies). Enriched (∼90%) CD25+CD4+ cells were cultured with anti-CD3 Ab (1 μg/mL), anti-CD28 Ab (1 μg/mL), and IL-2 (50 U/mL). Cells were then stimulated with OX40L-GST (5 μg/mL) or 4-1BBL–GST (5 μg/mL) or both, as indicated. When cells were stimulated by one ligand only, additional GST (5 μg/mL) was added. After 3 days of stimulation [3H]thymidine incorporation assay (16 hours of pulsing) was performed. Data indicate the average and the SD of triplicate samples. Two results obtained from independent experiments are shown. (B) CD8+ T cells were stimulated with anti-CD3 Ab (1 μg/mL) and anti-CD28 Ab (1 μg/mL) for 4 days. These preactivated cells were cultured with anti-CD3 Ab (1 μg/mL) and IL-2 (50 U/mL). Cells were simultaneously then stimulated with OX40L-GST (5 μg/mL) or 4-1BBL-GST (5 μg/mL) or both, as indicated. When cells were stimulated by one ligand only, additional GST (5 μg/mL) was added. After 3 days of stimulation, [3H]thymidine incorporation assay (16 hours of pulsing) was performed. Data indicate the average and the SD of triplicate samples. The result represents 3 experiments with similar results.
Cell growth inhibition of CD25+CD4+ T cells and CD8+ T cells by soluble OX40L and 4-1BBL. (A) CD4+ T cells were stimulated with anti-CD3 Ab (1 μg/mL) and anti-CD28 Ab (1 μg/mL) for 4 days. CD25+ cells were positively purified by anti-CD25 Ab and cell separation kit (StemCell Technologies). Enriched (∼90%) CD25+CD4+ cells were cultured with anti-CD3 Ab (1 μg/mL), anti-CD28 Ab (1 μg/mL), and IL-2 (50 U/mL). Cells were then stimulated with OX40L-GST (5 μg/mL) or 4-1BBL–GST (5 μg/mL) or both, as indicated. When cells were stimulated by one ligand only, additional GST (5 μg/mL) was added. After 3 days of stimulation [3H]thymidine incorporation assay (16 hours of pulsing) was performed. Data indicate the average and the SD of triplicate samples. Two results obtained from independent experiments are shown. (B) CD8+ T cells were stimulated with anti-CD3 Ab (1 μg/mL) and anti-CD28 Ab (1 μg/mL) for 4 days. These preactivated cells were cultured with anti-CD3 Ab (1 μg/mL) and IL-2 (50 U/mL). Cells were simultaneously then stimulated with OX40L-GST (5 μg/mL) or 4-1BBL-GST (5 μg/mL) or both, as indicated. When cells were stimulated by one ligand only, additional GST (5 μg/mL) was added. After 3 days of stimulation, [3H]thymidine incorporation assay (16 hours of pulsing) was performed. Data indicate the average and the SD of triplicate samples. The result represents 3 experiments with similar results.
Our data do not exclude the possibility that the inhibition of cell replication by the stimulation of OX40+4-1BB+ cells results from the synergistic signaling via OX40 and 4-1BB. However, no precedent for the inhibitory signaling by the synergy between TNFR family costimulatory receptors was reported before. To determine the origin of the inhibitory signaling, we may need tools that will allow us to distinguish between heterodimer receptors and non-heterodimer receptors. A monoclonal Ab specific for the OX40/4-1BB heterodimer could be useful to solve this issue, although such an Ab has not been yet successfully made in our laboratory. Originating from the OX40/4-1BB heterodimer or the synergy of OX40 and 4-1BB, our results in either case seem to present an intriguing novel inhibitory signaling mechanism that uses 2 different TNFR family receptors in activated T cells.
Although an inhibitory signaling mechanism in OX40/4-1BB–stimulated cells remains elusive, our data showed that the OX40/4-1BB heterodimer poorly associated with TRAF1. Significantly, TRAF1 is known as a unique member of the TRAF family protein possessing an antiapoptotic function. It has been previously shown that the overexpression of TRAF1 in transgenic mice reduces the antigen-induced apoptosis of CD8+ T cells.37 TRAF1 is also structurally different from other TRAF members and it can associate with TRAF2 as well as the caspase inhibitory proteins cIAP1 and cIAP2.38-40 A caspase-cleaved carboxyl-terminal fragment of TRAF1 was found to be an inhibitor of NF-κB activation by targeting the IκB protein serine kinase (IKK) complex. The caspase-mediated cleavage converts TRAF1 from a selective modulator of TNFR signaling to a general inhibitor of NF-κB activation.41 Accordingly, it is possible that the expression and stimulation of the OX40/4-1BB heterodimer result in an increased expression of carboxyl-terminal fragment of TRAF1 in cells following the caspase-mediated cleavage of TRAF1. As a result, the NF-κB pathway would be repressed, affecting the growth of cells, as well as it could sensitize the cells to apoptosis. Indeed, we have observed that the activation of NF-κB was reduced in OX40/4-1BB–stimulated OX40/4-1BB double-transfectant cells (Figure 7) and these cells were more sensitive to TNF-α–induced cell death (Figure 8). Currently, we are investigating the OX40/4-1BB double-positive cells via biochemical approaches to understand the molecular mechanisms underlying inhibitory signaling by NF-κB pathway targeting.
It is of interest to determine what cells present ligands for OX40 and 4-1BB on OX40/4-1BB T cells. As a most probable case, OX40L and 4-1BBL are both presented by the activated APCs. In fact, a human macrophage cell line, THP-1, can express OX40L and 4-1BBL messages following activation (A.O., unpublished data, December 2003). As an alternative possibility, it is noteworthy to notice that both OX40L and 4-1BBL were observed on activated T cells.42-45 Although more studies should be performed to determine T-cell subsets expressing OX40L and 4-1BBL, it is an intriguing scenario that an activated CD25+CD4+ T-cell subset (could be CD25+CD4+ regulatory T cells themselves) expresses both OX40L and 4-1BBL and inhibits the function of OX40/4-1BB double-positive activated T cells for the purpose of homeostasis. Collectively, the OX40/4-1BB heterodimer receptor may play a vital role in homeostasis of activated T cells by a mechanism in which the engagement with both ligands triggers a profound inhibitory signaling affecting cell replication and survival mechanism.
Finally, our data, along with others', attempts to explain the redundancy of the TNFR family of costimulatory receptors on activated T cells by showing (1) that the heterogeneity of signaling by each receptor is meritorious for regulating diverse T-cell subset function/differentiation; (2) an increase in specificity of T cell-APC pathways in cellular network; and (3) that the similarities among TNFR family members allow for complexes of receptors to generate de novo heterodimer signaling pathways. The OX40/4-1BB heterodimerization is a first example of what could be a tendency among the TNFR family to regulate homeostasis in T cells or in any other cell lineages.
Note added in proof. During the revision phase of the manuscript, 3 papers showed that the concomitant stimulation of OX40 and 4-1BB in mice enhances T-cell immune responses.46-48 The data may suggest that the signaling by OX40 and 4-1BB in mouse T cells is different from that of human T cells. Alternatively, the response of nonactivated T cells against the OX40/4-1BB stimulation may be different from that of previously activated OX40/4-1BB double-positive T cells.
Prepublished online as Blood First Edition Paper, June 7, 2005; DOI 10.1182/blood-2004-04-1622.
Supported by Canadian Institute of Health Research (CIHR).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Amgen for 4-1-BB–specific Ab. We also thank Dr Koichi Fuse for inspiring discussions.


![Figure 9. Cell growth inhibition of CD25+CD4+ T cells and CD8+ T cells by soluble OX40L and 4-1BBL. (A) CD4+ T cells were stimulated with anti-CD3 Ab (1 μg/mL) and anti-CD28 Ab (1 μg/mL) for 4 days. CD25+ cells were positively purified by anti-CD25 Ab and cell separation kit (StemCell Technologies). Enriched (∼90%) CD25+CD4+ cells were cultured with anti-CD3 Ab (1 μg/mL), anti-CD28 Ab (1 μg/mL), and IL-2 (50 U/mL). Cells were then stimulated with OX40L-GST (5 μg/mL) or 4-1BBL–GST (5 μg/mL) or both, as indicated. When cells were stimulated by one ligand only, additional GST (5 μg/mL) was added. After 3 days of stimulation [3H]thymidine incorporation assay (16 hours of pulsing) was performed. Data indicate the average and the SD of triplicate samples. Two results obtained from independent experiments are shown. (B) CD8+ T cells were stimulated with anti-CD3 Ab (1 μg/mL) and anti-CD28 Ab (1 μg/mL) for 4 days. These preactivated cells were cultured with anti-CD3 Ab (1 μg/mL) and IL-2 (50 U/mL). Cells were simultaneously then stimulated with OX40L-GST (5 μg/mL) or 4-1BBL-GST (5 μg/mL) or both, as indicated. When cells were stimulated by one ligand only, additional GST (5 μg/mL) was added. After 3 days of stimulation, [3H]thymidine incorporation assay (16 hours of pulsing) was performed. Data indicate the average and the SD of triplicate samples. The result represents 3 experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/6/10.1182_blood-2004-04-1622/6/m_zh80180583940009.jpeg?Expires=1767792053&Signature=eUq6g88NRtJPtkuVsdvZ1vH6zmb1nASQaJrpKbyj2xSy42r58r21fDjD4zqd5Lhb0mXsmo6A8Gs8V4o3M-XOcQK44WxS5CHJo7B34ukjyRHCMkKNc9nbkjJZKLDE21VTdK6tkdO78snV3EuguEloCd6P9L9ciR5fUWFBzwzALXEO2zMd6gAaT~VLxN~D883hC8PKZpl2jefRFqIcX3rNeCyzlvGXBJ0NK6MsJuzA2ncNYbijWJ3YS1B~PrWjoy~ckaVsTmI0H~VduHfX77waKiRj3-GCx~WUAE4WMpYVI2MQxWtJXRYsY-4emhw4rORRvTzs25duoe0V5ITv6Nd5-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal